Targeting IGF1/IGF1r signaling relieve pain and autophagic dysfunction in NTG-induced chronic migraine model of mice
- PMID: 39304806
- PMCID: PMC11414239
- DOI: 10.1186/s10194-024-01864-6
Targeting IGF1/IGF1r signaling relieve pain and autophagic dysfunction in NTG-induced chronic migraine model of mice
Erratum in
-
Correction: Targeting IGF1/IGF1r signaling relieve pain and autophagic dysfunction in NTG-induced chronic migraine model of mice.J Headache Pain. 2024 Oct 8;25(1):173. doi: 10.1186/s10194-024-01884-2. J Headache Pain. 2024. PMID: 39379801 Free PMC article. No abstract available.
Abstract
Background: Chronic migraine is a severe and common neurological disorder, yet its precise physiological mechanisms remain unclear. The IGF1/IGF1r signaling pathway plays a crucial role in pain modulation. Studies have shown that IGF1, by binding to its receptor IGF1r, activates a series of downstream signaling cascades involved in neuronal survival, proliferation, autophagy and functional regulation. The activation of these pathways can influence nociceptive transmission. Furthermore, alterations in IGF1/IGF1r signaling are closely associated with the development of various chronic pain conditions. Therefore, understanding the specific mechanisms by which this pathway contributes to pain is of significant importance for the development of novel pain treatment strategies. In this study, we investigated the role of IGF1/IGF1r and its potential mechanisms in a mouse model of chronic migraine.
Methods: Chronic migraine was induced in mice by repeated intraperitoneal injections of nitroglycerin. Mechanical and thermal hypersensitivity responses were assessed using Von Frey filaments and radiant heat, respectively. To determine the role of IGF1/IGF1r in chronic migraine (CM), we examined the effects of the IGF1 receptor antagonist ppp (Picropodophyllin) on pain behaviors and the expression of calcitonin gene-related peptide (CGRP) and c-Fos.
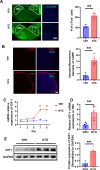
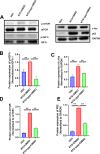
Result: In the nitroglycerin-induced chronic migraine model in mice, neuronal secretion of IGF1 is elevated within the trigeminal nucleus caudalis (TNC). Increased phosphorylation of the IGF1 receptor occurs, predominantly co-localizing with neurons. Treatment with ppp alleviated basal mechanical hypersensitivity and acute mechanical allodynia. Furthermore, ppp ameliorated autophagic dysfunction and reduced the expression of CGRP and c-Fos.
Conclusion: Our findings demonstrate that in the chronic migraine (CM) model in mice, there is a significant increase in IGF1 expression in the TNC region. This upregulation of IGF1 leads to enhanced phosphorylation of IGF1 receptors on neurons. Targeting and inhibiting this signaling pathway may offer potential preventive strategies for mitigating the progression of chronic migraine.
Keywords: Autophagy; Chronic migraine; IGF1; IGF1r; mTOR.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Feigin VL, Nichols E, Alam T, Bannick MS, Beghi E, Blake N, Culpepper WJ, Dorsey ER, Elbaz A, Ellenbogen RG et al (2019) Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of Disease Study 2016. Lancet Neurol 18:459–480. 10.1016/s1474-4422(18)30499-x - PMC - PubMed
-
- Schwedt TJ (2014) Chronic migraine. BMJ 348:g1416. 10.1136/bmj.g1416 - PubMed
-
- Dodick DW, Migraine (2018) Lancet 391:1315–1330. 10.1016/s0140-6736(18)30478-1 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

