An anti-eCIRP strategy for necrotizing enterocolitis
- PMID: 39304832
- PMCID: PMC11414128
- DOI: 10.1186/s10020-024-00935-3
An anti-eCIRP strategy for necrotizing enterocolitis
Abstract
Background: Necrotizing enterocolitis (NEC) is a severe gastrointestinal disease characterized by intestinal inflammation and injury, with high mortality risk. Extracellular cold-inducible RNA-binding protein (eCIRP) is a recently discovered damage-associated molecular pattern that propagates inflammation and tissue injury; however, the role of eCIRP in NEC remains unknown. We hypothesize that eCIRP exacerbates NEC pathogenesis and the novel eCIRP-scavenging peptide, milk fat globule-epidermal growth factor-factor VIII (MFG-E8)-derived oligopeptide 3 (MOP3), attenuates NEC severity, serving as a new therapeutic strategy to treat NEC.
Methods: Stool samples from premature neonates were collected prospectively and eCIRP levels were measured. Wild-type (WT) and CIRP-/- mouse pups were subjected to NEC utilizing a combination of hypoxia and hypercaloric formula orogastric gavage with lipopolysaccharide supplementation. In parallel, WT pups were treated with MOP3 or vehicle. Endpoints including NEC severity, intestinal injury, barrier dysfunction, lung injury, and overall survival were determined.
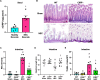
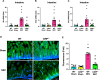
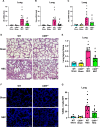
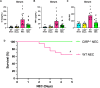
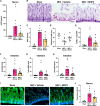
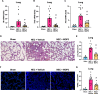
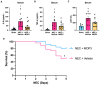
Results: Stool samples from NEC neonates had elevated eCIRP levels compared to healthy age-matched controls (p < 0.05). CIRP-/- pups were significantly protected from NEC severity, intestinal injury, bowel inflammation, intestinal barrier dysfunction, lung injury, and systemic inflammation. NEC survival was 100% for CIRP-/- pups compared to 65% for WT (p < 0.05). MOP3 treatment recapitulated the benefits afforded by CIRP-knockdown, preventing NEC severity, improving inflammatory profiles, and attenuating organ injury. MOP3 treatment improved NEC survival to 80% compared to 50% for vehicle treatment (p < 0.05).
Conclusions: eCIRP exacerbates NEC evidenced by protection with CIRP-deficiency and administration of MOP3, a CIRP-directed therapeutic, in a murine model. Thus, eCIRP is a novel target with human relevance, and MOP3 is a promising treatment for lethal NEC.
Keywords: Inflammation; MFG-E8; MOP3; Necrotizing enterocolitis; eCIRP.
© 2024. The Author(s).
Conflict of interest statement
The authors (CN, MA, and PW) are inventors of the pending PCT application on “A chimeric molecule to treat septic patients”; EFS ID: 47240580; Application No.: 63433789. Other authors report no financial competing interests.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

