Pumpkin seed oil: unveiling its potential in controlling inflammation and pathogenicity during experimental trichinellosis
- PMID: 39304848
- PMCID: PMC11414094
- DOI: 10.1186/s12917-024-04241-2
Pumpkin seed oil: unveiling its potential in controlling inflammation and pathogenicity during experimental trichinellosis
Abstract
Background: This study aimed to investigate the antiparasitic and anti-inflammatory potential of pumpkin seed oil in mice infected with Trichinella spiralis by demonstrating its impact on MMP-9 expression and pathogenesis during the intestinal and muscular phases.
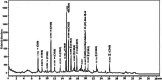
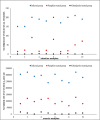
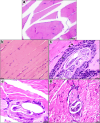
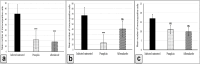
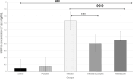
Results: In this study, 100 mice were divided into five groups: an infected group, a pumpkin seed oil-treated group (1.5 mg/kg BW, administered three times per week), an albendazole-treated group, a native control group, and a pumpkin oil control group. Gas chromatography-mass spectrometry analysis of the pumpkin seed oil revealed a broad spectrum of biologically active compounds. The pumpkin seed oil treatment led to a significant reduction in the parasite burden, with a 75% decrease in adult worms and a 66% decrease in encysted larvae. Additionally, the infected animals treated with pumpkin oil exhibited a marked reduction in intestinal inflammation, characterized by a progressive increase in goblet cells. The number of encysted larvae in the diaphragm and muscle tissues was also significantly decreased. Furthermore, pumpkin seed oil treatment significantly reduced MMP-9 levels in both intestinal and muscular tissues, highlighting its potential to attenuate inflammation.
Conclusion: These findings underscore the effectiveness of pumpkin seed oil as anti-inflammatory and antiparasitic agent.
Keywords: Trichinella; Anti-inflammatory; Antiparasitic; MMP-9; Pumpkin seed oil.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Nassef NE, Moharm IM, Atia AF, Brakat RM, Abou Hussien NM, Shamseldeen A. Therapeutic efficacy of chitosan nanoparticles loaded with albendazole on parenteral phase of experimental Trichinellosis. J Egypt Soc Parasitol. 2019;49(2):301–11. - DOI
-
- Speich B, Ali SM, Ame SM, Bogoch II, Alles R, Huwyler J, Albonico M, Hattendorf J, Utzinger J, Keiser J. Efficacy and safety of albendazole plus ivermectin, albendazole plus mebendazole, albendazole plus oxantel pamoate, and mebendazole alone against Trichuris trichiura and concomitant soil-transmitted helminth infections: a four-arm, randomised controlled trial. Lancet Infect Dis. 2015;15(3):277–84. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

