Role of the mechanotransductor PIEZO1 in megakaryocyte differentiation
- PMID: 39304946
- PMCID: PMC11415291
- DOI: 10.1111/jcmm.70055
Role of the mechanotransductor PIEZO1 in megakaryocyte differentiation
Abstract
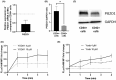
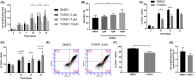
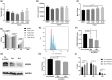
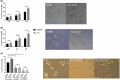
From haematopoietic stem cells to megakaryocytes (Mks), cells undergo various mechanical forces that affect Mk differentiation, maturation and proplatelet formation. The mechanotransductor PIEZO1 appears to be a natural candidate for sensing these mechanical forces and regulating megakaryopoiesis and thrombopoiesis. Gain-of-function mutations of PIEZO1 cause hereditary xerocytosis, a haemolytic anaemia associated with thrombotic events. If some functions of PIEZO1 have been reported in platelets, few data exist on PIEZO1 role in megakaryopoiesis. To address this subject, we used an in vitro model of Mk differentiation from CD34+ cells and studied step-by-step the effects of PIEZO1 activation by the chemical activator YODA1 during Mk differentiation and maturation. We report that PIEZO1 activation by 4 μM YODA1 at early stages of culture induced cytosolic calcium ion influx and reduced cell maturation. Indeed, CD41+CD42+ numbers were reduced by around 1.5-fold, with no effects on proliferation. At later stages of Mk differentiation, PIEZO1 activation promoted endomitosis and proplatelet formation that was reversed by PIEZO1 gene invalidation with a shRNA-PIEZO1. Same observations on endomitosis were reproduced in HEL cells induced into Mks by PMA and treated with YODA1. We provide for the first time results suggesting a dual role of PIEZO1 mechanotransductor during megakaryopoiesis.
Keywords: PIEZO1; megakaryocytes; proplatelet formation.
© 2024 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

