Freshwater snail-borne parasitic diseases in Africa
- PMID: 39304958
- PMCID: PMC11414283
- DOI: 10.1186/s41182-024-00632-1
Freshwater snail-borne parasitic diseases in Africa
Abstract
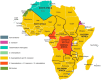
Background: Freshwater snails are the first obligatory intermediate hosts in the trematode life cycle. Several parasitic diseases transmitted by these snails are endemic in Africa, and their distribution closely follows that of the intermediate hosts. These diseases represent a major public health problem and cause significant socio-economic losses in Africa, particularly schistosomiasis and fascioliasis. In this review, we will describe the main roles of freshwater snails in the life cycle of trematode parasites, and the geographical distribution of these diseases in Africa. We will also discuss the different techniques for detecting parasitic infections in snails, as well as the various methods of controlling snails and the larval stages of parasites.
Methods: We carried out a literature search for articles dealing with parasitic diseases transmitted by freshwater snail hosts in Africa. The search was conducted in databases such as PubMed, Web of Science and Google Scholar using various search terms combined by Boolean operators. Our search was limited to peer-reviewed articles less than 10 years old. Articles published to date in the fields of control of parasitic diseases transmitted by freshwater snails were included. Results were presented in narrative and in table format.
Results: The results of the database search identified 1007 records. We included 84 studies in this review. These studies generally focused on freshwater snails and the diseases they transmit. We described the geographical distribution of 43 freshwater species belonging to nine snail families, as well as the parasites that infect them. Several methods for diagnosing parasites in their snail hosts have been described, including microscopic and molecular methods, as well as antibody and protein barcode-based techniques. Molluscicides have been described as the main strategy for snail control.
Conclusion: This study highlights several elements of knowledge about diseases transmitted by freshwater snails and their distribution. A good understanding of snail infection detection techniques and existing control methods is an essential component in adapting control strategies for these diseases.
Keywords: Africa; Diseases; Fasciolasis; Freshwater; Parasite; Schistosomiasis; Snail.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- OMS Agir Pour Réduire l’impact Mondial Des Maladies Tropicales Négligées : Premier Rapport de l’OMS Sur Les Maladies Tropicales Négligées. 2011.
-
- WHO Schistosomiasis. https://www.who.int/news-room/fact-sheets/detail/schistosomiasis. Accessed 15 Sept 2021.
-
- Diaw OT, Vassiliades G, Seye M, Sarr Y. Prolifération de Mollusques et Incidence Sur Les Trématodoses Dans La Région Du Delta et Du Lac de Guiers Après La Construction Du Barrage de Diama Sur Le Fleuve Sénégal. Rev Elev Med Vet Pays Trop. 1990;43:499–502. 10.19182/remvt.8773.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
