Aromaticity and Antiaromaticity Reversals between the Electronic Ground State and the Two Lowest Triplet States of Thiophene
- PMID: 39305154
- PMCID: PMC11747585
- DOI: 10.1002/cphc.202400758
Aromaticity and Antiaromaticity Reversals between the Electronic Ground State and the Two Lowest Triplet States of Thiophene
Abstract
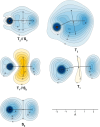
It is shown, by examining the variations in off-nucleus isotropic magnetic shielding around a molecule, that thiophene which is aromatic in its electronic ground state (S0) becomes antiaromatic in its lowest triplet state (T1) and then reverts to being aromatic in T2. Geometry relaxation has an opposite effect on the aromaticities of the ππ* vertical T1 and T2: The antiaromaticity of T1 is reduced whereas the aromaticity of T2 is enhanced. The shielding picture around T2 is found to closely resemble those around certain second singlet ππ* excited states (S2), for example, those of benzene and cyclooctatetraene, thought to be "strongly aromatic" because of their very negative nucleus-independent chemical shift (NICS) values. It is argued that while NICS values correctly follow the changes in aromaticity along the potential energy surface of a single electronic state, the use of NICS values for the purpose of quantitative comparisons between the aromaticities of different electronic states cannot be justified theoretically and should be avoided. "Strongly aromatic" S2 and T2 states should be referred to simply as "aromatic" because detailed comparisons between the properties of these states and those of the corresponding S0 states do not suggest higher levels of aromaticity.
Keywords: Baird's rule; Excited state aromaticity reversals; Nucleus-independent chemical shift; Off-nucleus shielding; Thiophene.
© 2024 The Authors. ChemPhysChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Baird N. C., J. Am. Chem. Soc. 1972, 94, 4941–4948.
-
- Durbeej B., Wang J., Oruganti B., ChemPlusChem 2018, 83, 958–967. - PubMed
-
- Oruganti B., Wang J., Durbeej B., Org. Lett. 2017, 19, 4818–4821. - PubMed
-
- Wang J., Oruganti B., Durbeej B., ChemPhotoChem 2019, 3, 450–460.
-
- Yamakado T., Takahashi S., Watanabe K., Matsumoto Y., Osuka A., Saito S., Angew. Chem. Int. Ed. 2018, 57, 5438–5443. - PubMed
LinkOut - more resources
Full Text Sources

