Synthesis and Biological Evaluation of Deoxycyclophellitols as Human Retaining β-Glucosidase Inhibitors
- PMID: 39305182
- PMCID: PMC11639629
- DOI: 10.1002/chem.202402988
Synthesis and Biological Evaluation of Deoxycyclophellitols as Human Retaining β-Glucosidase Inhibitors
Abstract
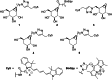
Cyclophellitol is a potent and selective mechanism-based retaining β-glucosidase inhibitor that has served as a versatile starting point for the development of activity-based glycosidase probes (ABPs). We developed routes of synthesis of eight mono- and dideoxycyclophellitols and cyclophellitol aziridines, the latter as ABPs carrying either a biotin or fluorophore linked to the aziridine nitrogen. We reveal the potency of these 24 compounds as inhibitors of the three human retaining β-glucosidases, GBA1, GBA2 and GBA3. We show that 3,6-dideoxy-β-galacto-cyclophellitol aziridine selectively captures GBA3 over GBA1 and GBA2 in extracts of cells overexpressing both GBA2 and GBA3. We also identify a probe that selectively labels GBA1 and GBA2 over GBA3 at lower concentrations. In sum, the here-presented studies reveal new chemistries to prepare chiral, substituted cyclitol epoxides and aziridines, add to the growing suite of cyclophellitols varying in configuration and substitution pattern, and yielded a reagent that may find use to investigate the physiological role and therapeutic relevance of the most elusive of the three retaining β-glucosidases: GBA3.
Keywords: Carbasugar; Cyclophellitol; Deoxygenation; Glucosidase; Inhibitors and activity-based probes (ABPs).
© 2024 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Aerts J. M. F. G., Kuo C. L., Lelieveld L. T., Boer D. E. C., van der Lienden M. J. C., Overkleeft H. S., Artola M., Curr. Opin. Chem. Biol. 2019, 53, 204–215. - PubMed
-
- Ying J. F., Zhang Y. N., Song S. S., Hu Z. M., He X. L., Pan H. Y., Zhang C. W., Wang H. J., Li W. F., Mou X. Z., Neoplasma 2020, 67, 1139–1145. - PubMed
-
- Braun H., Legler G., Deshusses J., Semenza G., Biochim. Biophys. Acta Enzymol. 1977, 483, 135–140. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

