Changes in conjunctival mononuclear phagocytes and suppressive activity of regulatory macrophages in desiccation induced dry eye
- PMID: 39306240
- PMCID: PMC11984642
- DOI: 10.1016/j.jtos.2024.09.003
Changes in conjunctival mononuclear phagocytes and suppressive activity of regulatory macrophages in desiccation induced dry eye
Abstract
Purpose: To evaluate the effects of dry eye on conjunctival immune cell number and transcriptional profiles with attention to mononuclear phagocytes.
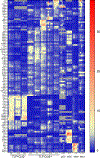
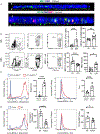
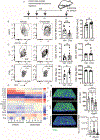
Methods: Expression profiling was performed by single-cell RNA sequencing on sorted conjunctival immune cells from non-stressed and C57BL/6 mice subjected to desiccating stress (DS). Monocle 3 modeled cell trajectory, scATAC-seq assessed chromatin accessibility and IPA identified canonical pathways. Inflammation and goblet cells were measured after depletion of MRC1+ MΦs with mannosylated clodronate liposomes.
Results: Mononuclear phagocytes (monocytes, MΦs, DCs) comprised 72 % of immune cells and showed the greatest changes with DS. Distinct DS induced gene expression patterns were seen in phagocytes classified by expression of Ccr2 and [Timd4, Lyve1, Folr2 (TLR)]. Expression of phagocytosis/efferocytosis genes increased in TLF+CCR2- MΦs. Monocytes showed the highest expression of Ace, Cx3cr1, Vegfa, Ifngr1,2, and Stat1 and TLF-CCR2+ cells expressed higher levels of inflammatory mediators (Il1a, Il1b, Il1rn, Nfkb1, Ccl5, MHCII, Cd80, Cxcl10, Icam1). A trajectory from monocyte precursors branched to terminate in regulatory MΦs or in mDCs via transitional MΦ and cDC clusters. Activated pathways in TLF+ cells include phagocytosis, PPAR/RXRα activation, IL-10 signaling, alternate MΦ activation, while inflammatory pathways were suppressed. Depletion of MRC1+ MΦs increased IL-17 and IFN-γ expression and cytokine-expressing T cells, reduced IL-10 and worsened goblet loss.
Conclusions: Dryness stimulates distinct gene expression patterns in conjunctival phagocytes, increasing expression of regulatory genes in TLF+ cells regulated in part by RXRα, and inflammatory genes in CCR2+ cells. Regulatory MΦs depletion worsens DS induced inflammation and goblet cell loss.
Keywords: Alternative activation; Conjunctiva; Dry eye; Innate immunity; Macrophage; Phagocyte.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest Patent BLG 22–028; “RXR Agonists in Eye Disorders”; BAYM. P0358WO was filed by Baylor College of Medicine on 1/29/23 and lists SCP and JA as coinventors.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

