Comparative early effectiveness across 14 PsA drugs and 5 classes of PsA treatment: 3-month results from the PRO-SPIRIT study
- PMID: 39306343
- PMCID: PMC11418525
- DOI: 10.1136/rmdopen-2024-004318
Comparative early effectiveness across 14 PsA drugs and 5 classes of PsA treatment: 3-month results from the PRO-SPIRIT study
Abstract
Background: The psoriatic arthritis (PsA) Observational Study of Persistence of Treatment (PRO-SPIRIT) assesses effectiveness and persistence of real-world PsA treatments. Ixekizumab (IXE) is an interleukin (IL)-17A inhibitor (i) (IL-17Ai), approved for the treatment of adult PsA.
Methods: The aim of this predefined interim analysis was to report baseline characteristics along with early (3-month) descriptive and comparative real-world effectiveness in patients with PsA prescribed with advanced treatment including IL-17Ai; IXE or secukinumab (SEC), IL-12/23i, IL-23i, tumour necrosis factor (TNFi) or Janus kinase (JAKi).
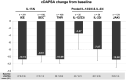
Results: 1192 patients across 6 countries were analysed. At baseline, patients receiving IXE had longer disease duration and higher previous biological/targeted-synthetic disease-modifying antirheumatic drugs experience than patients starting TNFi and SEC 150, and less concomitant conventional-synthetic DMARD use than TNFi and JAKi. Comparative analyses at 3 months showed that: (a) versus TNFi, IXE exhibited similar improvement in clinical Disease Activity in PsA (cDAPSA) but significantly greater improvement in body surface area affected by psoriasis (BSA) and global assessments (physician GA, patient GA (PatGA)); (b) versus IL-12/23i and IL-23i (pooled), IXE showed significantly greater improvement in cDAPSA and PatGA; (c) IXE was as fast as JAKi in improving joint disease activity. Ad hoc analysis indicated that more patients with active psoriasis (BSA ≥3%) achieved minimal disease activity with IXE than JAKi or IL-12/23i. The responses to SEC varied by dosage.
Conclusions: This study confirms the rapid 3-month effectiveness of IXE on joint disease activity-as fast as TNFi and JAKi (cDAPSA), and exceeding IL-12/23i and IL-23i-along with clear benefits to skin.
Keywords: arthritis, psoriatic; health-related quality of life; interleukin-17; patient reported outcome measures; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LEK has received honoraria or fees for serving as a speaker or consultant from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Eli Lilly and Company, Gilead, GSK, Janssen, Merck, Novartis, Pfizer and UCB. He has received investigator-initiated study grants from AbbVie, Biogen, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB. KJN and MN are employees and minor shareholders of Eli Lilly and Company. JM has received grants, speaker honoraria or travel support, or participated on an advisory board for AbbVie, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Fresenius Kabi, Galapagos, Medac, Mylan, Novartis, Pfizer, Roche Chugaï, Sanofi and Viatris. EL has received speaker honoraria from AbbVie, Eli Lilly and Company, Janssen, Novartis and UCB. WT has received grants, consulting fees, speaker honoraria and/or travel support from AbbVie, Eli Lilly and Company, GSK, Janssen, Novartis, Ono-Pharma, Pfizer and UCB. RA has received consulting fees, speaker honoraria and/or travel support from AbbVie, Amgen, Bristol Myers Squibb, Celltrion, Eli Lilly and Company, Galapagos, Gilead, Janssen, Mylan, Novartis, Pfizer, Roche Chugaï, Viatris and UCB. VC has received grants, royalties or consulting fees, or had a leadership role in, AbbVie, Amgen, Bristol Myers Squibb, Canadian Psoriasis Network, Eli Lilly and Company, Fresenius Kabi, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), Janssen, Novartis, UCB and University Health Network. AMF has received speaker honoraria, payment for expert testimony and/or travel support from AbbVie, Eli Lilly and Company, Janssen, Pfizer, Novartis and UCB. BZ, DK and TH are employees and minor shareholders of Eli Lilly and Company. NG has received grants, consulting fees, speaker honoraria, travel support or equipment/services, or participated on an advisory board for AbbVie, AstraZeneca, Eli Lilly and Company, Galapagos, Janssen, Novartis and UCB. AK, WF and IdlT are employees and minor shareholders of Eli Lilly and Company. DGMcG has received consulting fees, speaker honoraria, research grant support and/or travel support from AbbVie, Almiral, Bristol Myers Squibb, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB.
Figures


References
-
- Gossec L, Smolen J. EULAR recommendations for the management of psoriatic arthritis: 2023 update. [PowerPoint presentation]. European Alliance of Associations for Rheumatology (EULAR). Presented June 3, 2023 n.d.
-
- US FDA TALTZ (ixekizumab) injection, for subcutaneous use: US prescribing information. 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2016/125521s000lbl.pdf Available.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
