56Fe-ion Exposure Increases the Incidence of Lung and Brain Tumors at a Similar Rate in Male and Female Mice
- PMID: 39307527
- PMCID: PMC11608577
- DOI: 10.1667/RADE-24-00004.1
56Fe-ion Exposure Increases the Incidence of Lung and Brain Tumors at a Similar Rate in Male and Female Mice
Abstract
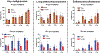
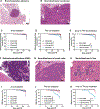
The main deterrent to long-term space travel is the risk of Radiation Exposure Induced Death (REID). The National Aeronautics and Space Administration (NASA) has adopted Permissible Exposure Levels (PELs) to limit the probability of REID to 3% for the risk of death due to radiation-induced carcinogenesis. The most significant contributor to current REID estimates for astronauts is the risk of lung cancer. Recently updated lung cancer estimates from Japan's atomic bomb survivors showed that the excess relative risk of lung cancer by age 70 is roughly fourfold higher in females compared to males. However, whether sex differences may impact the risk of lung cancer due to exposure to high charge and energy (HZE) radiation is not well studied. Thus, to evaluate the impact of sex differences on the risk of solid cancer development after HZE radiation exposure, we irradiated Rbfl/fl, Trp53fl/+ male and female mice infected with Adeno-Cre with various doses of 320 kVp X rays or 600 MeV/n 56Fe ions and monitored them for any radiation-induced malignancies. We conducted complete necropsy and histopathology of major organs on 183 male and 157 female mice after following them for 350 days postirradiation. We observed that lung adenomas/carcinomas and esthesioneuroblastomas (ENBs) were the most common primary malignancies in mice exposed to X rays and 56Fe ions, respectively. In addition, 1 Gy 56Fe-ion exposure compared to X-ray exposure led to a significantly increased incidence of lung adenomas/carcinomas (P = 0.02) and ENBs (P < 0.0001) in mice. However, we did not find a significantly higher incidence of any solid malignancies in female mice as compared to male mice, regardless of radiation quality. Furthermore, gene expression analysis of ENBs suggested a distinct gene expression pattern with similar hallmark pathways altered, such as MYC targets and MTORC1 signaling, in ENBs induced by X rays and 56Fe ions. Thus, our data revealed that 56Fe-ion exposure significantly accelerated the development of lung adenomas/carcinomas and ENBs compared to X rays, but the rate of solid malignancies was similar between male and female mice, regardless of radiation quality.
© 2024 by Radiation Research Society. All rights of reproduction in any form reserved.
Figures


Update of
-
56 Fe ion exposure increases the incidence of lung and brain tumors at a similar rate in male and female mice.bioRxiv [Preprint]. 2023 Jun 8:2023.06.06.543754. doi: 10.1101/2023.06.06.543754. bioRxiv. 2023. Update in: Radiat Res. 2024 Nov 1;202(5):734-744. doi: 10.1667/RADE-24-00004.1. PMID: 37333373 Free PMC article. Updated. Preprint.
References
-
- Cucinotta F, Durante M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. Lancet Oncol 2006; 7(5):431–5. - PubMed
-
- Schimmerling W, Cucinotta FA, Wilson JW. Radiation risk and human space exploration. Adv Space Res 2003; 31(1):27–34. - PubMed
-
- Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nat Rev Cancer 2008; 8(6):465–72. - PubMed
-
- Cucinotta FA, Schimmerling W, Wilson JW, Peterson LE, Badhwar GD, Saganti PB, et al. Space radiation cancer risks and uncertainties for Mars missions. Radiat Res 2001; 156(5 Pt 2):682–8. - PubMed
-
- Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M, et al. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res 2007; 168(1):1–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

