Dietary fibers boost gut microbiota-produced B vitamin pool and alter host immune landscape
- PMID: 39307855
- PMCID: PMC11418204
- DOI: 10.1186/s40168-024-01898-7
Dietary fibers boost gut microbiota-produced B vitamin pool and alter host immune landscape
Abstract
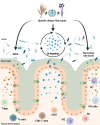
Background: Dietary fibers can alter microbial metabolic output in support of healthy immune function; however, the impact of distinct fiber sources and immunomodulatory effects beyond short-chain fatty acid production are underexplored. In an effort to discern the effects of diverse fibers on host immunity, we employed five distinct rodent diets with varying fiber content and source in specific-pathogen-free, gnotobiotic (containing a 14-member synthetic human gut microbiota), and germ-free mice.
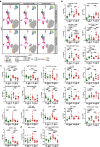
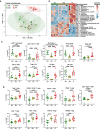
Results: Broad-scale metabolomics analysis of cecal contents revealed that fiber deprivation consistently reduced the concentrations of microbiota-produced B vitamins. This phenomenon was not always explained by reduced biosynthesis, rather, metatranscriptomic analyses pointed toward increased microbial usage of certain B vitamins under fiber-free conditions, ultimately resulting in a net reduction of host-available B vitamins. Broad immunophenotyping indicated that the local gut effector immune populations and activated T cells accumulate in a microbiota-dependent manner. Supplementation with the prebiotic inulin recovered the availability of microbially produced B vitamins and restored immune homeostasis.
Conclusions: Our findings highlight the potential to use defined fiber polysaccharides to boost microbiota-derived B vitamin availability in an animal model and to regulate local innate and adaptive immune populations of the host. Video abstract.
Keywords: B vitamins; Dietary fiber; Mass cytometry; Microbiome.
© 2024. The Author(s).
Conflict of interest statement
M.S.D. works as a consultant and an advisory board member at Theralution GmbH, Germany. S.F. is a founder and CEO of Metagen, Inc., Japan, focused on the design and control of the gut environment for human health.
Figures



References
-
- Ansaldo E, Farley TK, Belkaid Y. Control of immunity by the microbiota. Ann Rev Immunol. 2021;39:449–79. 10.1146/annurev-immunol-093019-112348. - PubMed
-
- Wolter M, Grant ET, Boudaud M, Steimle A, Pereira G V, Martens EC, et al. Leveraging diet to engineer the gut microbiome. Nat Rev Gastroenterol Hepatol. 2021;18:885–902. 10.1038/s41575-021-00512-7. - PubMed
-
- Gentile CL, Weir TL. The gut microbiota at the intersection of diet and human health. Science. 2018;362:776–80. 10.1126/science.aau5812. - PubMed
-
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. 10.1038/nature25973. - PubMed
-
- Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–15. 10.1016/j.chom.2018.05.012. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

