Impact of short-term proton pump inhibitors vs . histamine-2 receptor antagonists on gut microbiota in patients with acute coronary syndrome: A multicenter randomized trial
- PMID: 39307932
- PMCID: PMC11882281
- DOI: 10.1097/CM9.0000000000003148
Impact of short-term proton pump inhibitors vs . histamine-2 receptor antagonists on gut microbiota in patients with acute coronary syndrome: A multicenter randomized trial
Abstract
Background: Several randomized controlled studies have suggested that the prophylactic use of proton pump inhibitors (PPIs) in intensive care unit (ICU) patients could not reduce the incidence of gastrointestinal bleeding (GIB) and may increase adverse events such as intestinal infection and pneumonia. Gut microbiota may play a critical role in the process. PPIs have been widely prescribed for GIB prophylaxis in patients with acute coronary syndrome (ACS). This study aimed to determine the short-term effects of PPI and histamine-2 receptor antagonist (H2RA) treatment on gut microbiota of ACS patients.
Methods: The study was designed as a single-blind, multicenter, three-parallel-arm, randomized controlled trial conducted at three centers in Beijing, China. We enrolled ACS patients at low-to-medium risk of GIB and randomized (2:2:1) them to either PPI ( n = 40), H2RA ( n = 31), or control group ( n = 21). The primary outcomes were the alterations in gut microbiota after 7 days of acid suppressant therapy. Stool samples were collected at baseline and 7 days and analyzed by 16S ribosomal RNA (rRNA) gene sequencing.
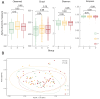
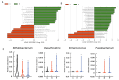
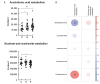
Results: There were no significant changes in the diversity of gut microbiota after the short-term use of acid suppressants, but the abundance of Fusobacterium significantly increased and that of Bifidobacterium significantly decreased, especially in PPI users. In addition, the abundance of some pathogenic bacteria, including Enterococcus and Desulfovibrio, was significantly elevated in the PPI users. The fecal microbiota of the PPI users included more arachidonic acid metabolism than that of control group.
Conclusions: PPIs may increase the risk of infection by adversely altering gut microbiota and elevating arachidonic acid metabolism, which may produce multiple proinflammatory mediators. For ACS patients at low-to-medium risk of GIB, sufficient caution should be paid when acid-suppressant drugs are prescribed, especially PPIs.
Registration: www.chictr.org.cn (ChiCTR2000029552).
Keywords: Acute coronary syndrome; Gut microbiota; Histamine-2 receptor antagonists; Proton pump inhibitors.
Copyright © 2024 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
None.
Figures


References
-
- Valgimigli M Bueno H Byrne RA Collet JP Costa F Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg 2018;53:34–78. doi: 10.1093/ejcts/ezx334. - PubMed
-
- Généreux P Giustino G Witzenbichler B Weisz G Stuckey TD Rinaldi MJ, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol 2015;66:1036–1045. doi: 10.1016/j.jacc.2015.06.1323. - PubMed
-
- Krag M Perner A Wetterslev J Wise MP Borthwick M Bendel S, et al. Stress ulcer prophylaxis in the intensive care unit: An international survey of 97 units in 11 countries. Acta Anaesthesiol Scand 2015;59:576–585. doi: 10.1111/aas.12508. - PubMed
-
- Sehested TSG Carlson N Hansen PW Gerds TA Charlot MG Torp-Pedersen C, et al. Reduced risk of gastrointestinal bleeding associated with proton pump inhibitor therapy in patients treated with dual antiplatelet therapy after myocardial infarction. Eur Heart J 2019;40:1963–1970. doi: 10.1093/eurheartj/ehz104. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

