The Immune-Related 27-Gene Signature DetermaIO Predicts Response to Neoadjuvant Atezolizumab plus Chemotherapy in Triple-Negative Breast Cancer
- PMID: 39308141
- PMCID: PMC11528202
- DOI: 10.1158/1078-0432.CCR-24-0149
The Immune-Related 27-Gene Signature DetermaIO Predicts Response to Neoadjuvant Atezolizumab plus Chemotherapy in Triple-Negative Breast Cancer
Abstract
Purpose: We assessed the 27-gene RT-qPCR-based DetermaIO assay and the same score calculated from RNA sequencing (RNA-seq) data as predictors of sensitivity to immune checkpoint therapy in the neoTRIPaPDL1 randomized trial that compared neoadjuvant carboplatin/nab-paclitaxel chemotherapy (CT) plus atezolizumab with CT alone in stage II/III triple-negative breast cancer. We also assessed the predictive function of the immuno-oncology (IO) score in expression data of patients treated with pembrolizumab plus paclitaxel (N = 29) or CT alone (N = 56) in the I-SPY2 trial.
Experimental design: RNA-seq data were obtained from pretreatment core biopsies from 242 (93.8%) of the 258 patients in the per-protocol-population. The DetermaIO RT-qPCR test, performed in the CAP/CLIA-accredited laboratory of Oncocyte Corp., was available for 220 patients (85.3%). A previously established threshold was used to assign DetermaIO-positive versus DetermaIO-negative status. Publicly available microarray data were used from I-SPY2.
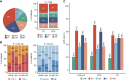
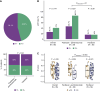
Results: IO scores calculated from RNA-seq and RT-qPCR data were highly concordant. In neoTRIPaPDL1, DetermaIO-positive cancers (N = 92, 41.8%) had pathologic complete response (pCR) rates of 69.8% and 46.9% in the CT + atezolizumab and CT arms, respectively. In DetermaIO-negative cases, pCR rates were similar in both arms (44.6% vs. 49.2%; interaction test P = 0.04). PDL1 protein expression and stromal tumor-infiltrating lymphocyte count were not predictive of differential benefit from atezolizumab. In I-SPY2, IO-positive cancers (45.9%) had pCR rates of 85.7% and 16%, with and without immunotherapy, respectively. In IO-negative cancers, pCR rates were 46.7% versus 16.1%.
Conclusions: DetermaIO identified patients who benefited from neoadjuvant immunotherapy resulting in improved pCR rate, independently of PDL1.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
C.-S. Huang reports grants, personal fees, and non-financial support from AstraZeneca, Novartis, OBI Pharma, Pfizer, Daiichi Sankyo, EirGenix, Eli Lilly, and from Roche during the conduct of the study; grants from Aston Sci. and Seagen; grants and personal fees from Gilead; and grants and non-financial support from MSD outside the submitted work. D. Egle reports personal fees from AstraZeneca, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, Menarini, and Seagen; personal fees and non-financial support from Daiichi Sankyo; and grants and personal fees from Sirius Medical during the conduct of the study. B. Bermejo reports personal fees from MSD, Roche, Lilly, and Novartis, and other support from Pfizer and Gilead outside the submitted work. C. Zamagni reports grants from Michelangelo Tech during the conduct of the study and from AbbVie, Array BioPharma, and Synthon outside the submitted work; grants, personal fees, and non-financial support from AstraZeneca, Daiichi Sankyo, Gilead, GSK, MSD, Novartis, Pfizer, and Roche; and personal fees and non-financial support from Eisai, Exact Sciences, Lilly, and Seagen. R.S. Seitz reports personal fees from Insight Genetics Inc. during the conduct of the study and from Gene Capture and BioGenerator Ventures outside the submitted work; in addition, R.S. Seitz reports a patent for WO2022031630A1 pending. T.J. Nielsen reports personal fees from Oncocyte Corporation during the conduct of the study as well as a patent for Classifying Tumors and Predicting Responsiveness pending to Oncocyte Corporation. M. Thill reports personal fees and non-financial support from Roche during the conduct of the study; personal fees from Agendia, Amgen, AstraZeneca, ClearCut, Daiichi Sankyo, Eisai, Gilead, Hexal, MSD, Grünengthal, GSK, Onkowissen, Jörg Eickeler, Organon, Pfizer, Seagen, Pierre Fabre, Sirius Medical, Sysmex, Stemline, Vifor, ZP Therapeutics, and StreamedUp; grants and personal fees from Exact Science, NeoDynamics, and Novartis; and grants, personal fees, and non-financial support from PFM Medical outside the submitted work. A. Antón-Torres reports personal fees from Lilly, Pfizer, Seagen, Novartis, Roche, and Daiichi Sankyo outside the submitted work. B.L. Schweitzer reports personal fees from Oncocyte Corporation during the conduct of the study and outside the submitted work, as well as a patent for WO2022031630A1 pending to Oncocyte Corporation. D.T. Ross reports personal fees from Oncocyte Inc. during the conduct of the study as well from TwinStrand Biosciences Inc. outside the submitted work. R. Greil reports other support from Celgene, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, BMS, MSD, Sandoz, AbbVie, Gilead, Daiichi Sankyo, Sanofi, Novo Nordisk, and Lilly outside the submitted work. M. Colleoni reports grants from Roche outside the submitted work. L. Del Mastro reports grants and personal fees from Eli Lilly, Novartis, GSK, and Seagen; grants, personal fees, and non-financial support from Roche, Daiichi Sankyo, AstraZeneca, and Gilead; personal fees from Pierre Fabre, Pfizer, MSD, Eisai, Agendia, Menarini/Stemline, Ipsen, Olema, and Exact Sciences; and grants from AIRC outside the submitted work. L. Pusztai reports personal fees and other support from Merck, AstraZeneca, and Pfizer, as well as personal fees from Oncocyte outside the submitted work. G. Viale reports personal fees from Roche, AstraZeneca, Daiichi Sankyo, Gilead, Pfizer, and Agilent outside the submitted work. L. Gianni reports grants from the Breast Cancer Research Foundation during the conduct of the study, as well as personal fees from AstraZeneca, Roche, Pfizer, Zymeworks, Artemida Pharma Ltd, Daiichi Sankyo, BeiGene, Revolution Medicines, Synaffix, Menarini Ricerche, Amgen, Biomedical Insights Inc., and Denali Therapeutics Inc. outside the submitted work; L. Gianni also reports a patent for European Patent Application Nos. 12195182.6 and 12196177.5 issued. G. Bianchini reports grants from Fondazione AIRC per la Ricerca sul Cancro during the conduct of the study; personal fees from Roche, Eli Lilly, Pfizer, AstraZeneca, MSD, Daiichi Sankyo, EISAI, Menarini/Stemline, Exact Science, Seagen, Agendia, and Novartis; and grants and personal fees from Gilead outside the submitted work. No disclosures were reported by the other authors.
Figures

References
-
- Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2018;29:84–91. - PubMed
-
- Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 2018;378:158–68. - PubMed
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. . Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007;13:4429–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

