A ROS-Responsive Lipid Nanoparticles Release Multifunctional Hydrogel Based on Microenvironment Regulation Promotes Infected Diabetic Wound Healing
- PMID: 39308241
- PMCID: PMC11578374
- DOI: 10.1002/advs.202403219
A ROS-Responsive Lipid Nanoparticles Release Multifunctional Hydrogel Based on Microenvironment Regulation Promotes Infected Diabetic Wound Healing
Abstract
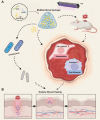
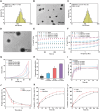
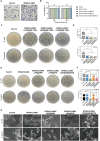
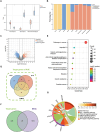
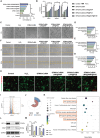
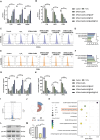
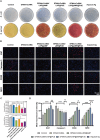
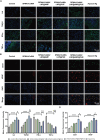
The continuous imbalance of the diabetic wound microenvironment is an important cause of chronic nonhealing, which manifests as a vicious cycle between excessive accumulation of reactive oxygen species (ROS) and abnormal healing. Regulating the microenvironment by suppressing wound inflammation, oxidative stress, and bacterial infection is a key challenge in treating diabetic wounds. In this study, ROS-responsive hydrogels are developed composed of silk fibroin methacrylated (SFMA), modified collagen type III (rCol3MA), and lipid nanoparticles (LNPs). The newly designed hydrogel system demonstrated stable physicochemical properties and excellent biocompatibility. Moreover, the release of antimicrobial peptide (AMP) and puerarin (PUE) demonstrated remarkable efficacy in eradicating bacteria, regulating inflammatory responses, and modulating vascular functions. This multifunctional hydrogel is a simple and efficient approach for the treatment of chronic diabetic infected wounds and holds tremendous potential for future clinical applications.
Keywords: antibacterial; antioxidation; anti‐inflammation; diabetic wound; multifunctional hydrogel.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
