A non-genetic model of vascular shunts informs on the cellular mechanisms of formation and resolution of arteriovenous malformations
- PMID: 39308243
- PMCID: PMC11629978
- DOI: 10.1093/cvr/cvae160
A non-genetic model of vascular shunts informs on the cellular mechanisms of formation and resolution of arteriovenous malformations
Abstract
Aims: Arteriovenous malformations (AVMs), a disorder characterized by direct shunts between arteries and veins, are associated with genetic mutations. However, the mechanisms leading to AV shunt formation and how shunts can be reverted are poorly understood.
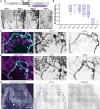
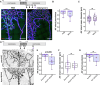
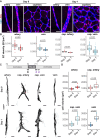
Methods and results: Here, we report that oxygen-induced retinopathy (OIR) protocol leads to the consistent and stereotypical formation of AV shunts in non-genetically altered mice. OIR-induced AV shunts show all the canonical markers of AVMs. Genetic and pharmacological interventions demonstrated that changes in the volume of venous endothelial cells (EC)-hypertrophic venous cells-are the initiating step promoting AV shunt formation, whilst EC proliferation or migration played minor roles. Inhibition of the mTOR pathway prevents pathological increases in EC volume and significantly reduces the formation of AV shunts. Importantly, we demonstrate that ALK1 signalling cell-autonomously regulates EC volume in pro-angiogenic conditions, establishing a link with hereditary haemorrhagic telangiectasia-related AVMs. Finally, we demonstrate that a combination of EC volume control and EC migration is associated with the regression of AV shunts.
Conclusion: Our findings highlight that an increase in the EC volume is the key mechanism driving the initial stages of AV shunt formation, leading to asymmetric capillary diameters. Based on our results, we propose a coherent and unifying timeline leading to the fast conversion of a capillary vessel into an AV shunt. Our data advocate for further investigation into the mechanisms regulating EC volume in health and disease as a way to identify therapeutic approaches to prevent and revert AVMs.
Keywords: Angiogenesis; Arteriovenous malformations; Cell volume; Oxygen-induced retinopathy.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures





References
-
- Alaraj A, Shakur SF, Amin-Hanjani S, Mostafa H, Khan S, Aletich VA, Charbel FT. Changes in wall shear stress of cerebral arteriovenous malformation feeder arteries after embolization and surgery. Stroke 2015;46:1216–1220. - PubMed
-
- Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, Khyzha N, DiStefano PV, Suutarinen S, Kiehl TR, Mendes Pereira V, Herman AM, Krings T, Andrade-Barazarte H, Tung T, Valiante T, Zadeh G, Tymianski M, Rauramaa T, Ylä-Herttuala S, Wythe JD, Antonarakis SE, Frösen J, Fish JE, Radovanovic I. Somatic activating KRAS mutations in arteriovenous malformations of the brain. N Engl J Med 2018;378:250–261. - PMC - PubMed
-
- Dupuis-Girod S, Bailly S, Plauchu H. Hereditary hemorrhagic telangiectasia: from molecular biology to patient care. J Thromb Haemost 2010;8:1447–1456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

