PET/MRI in paediatric inflammatory bowel disease - a prospective accuracy study
- PMID: 39308430
- PMCID: PMC11650528
- DOI: 10.1111/cpf.12903
PET/MRI in paediatric inflammatory bowel disease - a prospective accuracy study
Abstract
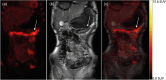
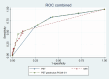
Cross-sectional imaging supplements endoscopy in detecting disease manifestations in inflammatory bowel diseases (IBD). This study aimed to evaluate the accuracy of PET/MRI in a paediatric population suspected of IBD. This prospective study consecutively included patients aged 8-17 years under diagnostic evaluation for IBD. Forty-three patients underwent a PET/MRI scan and subsequent ileocolonoscopy, of whom 26 patients diagnosed with IBD participated in a follow-up scan, hereof 19 with Crohn's disease (CD), five with Ulcerative colitis and two with unclassified IBD. The results of PET alone, MRI alone, and PET/MRI combined were compared to a reference standard of endoscopy and histopathology. Of the 208 intestinal segments analysed, 109 showed inflammation, and 99 had no inflammation. In the per-segment analysis PET had a sensitivity of 0.83 (95% CI 0.73-0.93), specificity of 0.59 (95% CI 0.47-0.71), and area under the receiver operating characteristic curve (AUROC) of 0.73 (95% CI 0.67-0.80). MRI had a sensitivity of 0.52 (95% CI 0.41-0.64), specificity 0.89 (95% CI 0.82-0.96), and AUROC of 0.72 (95% CI 0.66-0.77). PET/MRI had a sensitivity of 0.83 (95% CI 0.74-0.94), specificity of 0.57 (95% CI 0.44-0.69), and AUROC of 0.77 (95% CI 0.71-0.84). At follow-up, PET and MRI scores decreased, and the change in MRI was able to identify patients with a clinical response. The accuracy of the PET/MRI scan in detecting inflammation in the terminal ileum and colon was moderate and not superior to either modality alone. With technological advances and combined reading, PET/MRI may still be valuable in selected cases.
Keywords: Crohn's disease; Positron emission tomography; Ulcerative colitis; adolescents; children; magnetic resonance imaging.
© 2024 The Author(s). Clinical Physiology and Functional Imaging published by John Wiley & Sons Ltd on behalf of Scandinavian Society of Clinical Physiology and Nuclear Medicine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Bossuyt, P.M. , Reitsma, J.B. , Bruns, D.E. , Gatsonis, C.A. , Glasziou, P.P. , Irwig, L. et al. (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. Clinical Chemistry, 61, 1446–1452. - PubMed
-
- Chen, W. , Liu, L. , Li, Y. , Li, S. , Li, Z. , Zhang, W. et al. (2022) Evaluation of pediatric malignancies using total‐body PET/CT with half‐dose [(18)F]‐FDG. European Journal of Nuclear Medicine and Molecular Imaging, 49, 4145–4155. - PubMed
-
- Daperno, M. , D'Haens, G. , Van Assche, G. , Baert, F. , Bulois, P. , Maunoury, V. et al. (2004) Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES‐CD. Gastrointestinal Endoscopy, 60, 505–512. - PubMed
Publication types
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

