In-host modeling of dengue virus and non-structural protein 1 and the effects of ivermectin in patients with acute dengue fever
- PMID: 39308445
- PMCID: PMC11646939
- DOI: 10.1002/psp4.13233
In-host modeling of dengue virus and non-structural protein 1 and the effects of ivermectin in patients with acute dengue fever
Abstract
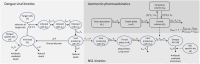
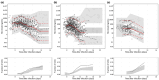
The increased incidence of dengue poses a substantially global public health challenge. There are no approved antiviral drugs to treat dengue infections. Ivermectin, an old anti-parasitic drug, had no effect on dengue viremia, but reduced the dengue non-structural protein 1 (NS1) in a clinical trial. This is potentially important, as NS1 may play a causal role in the pathogenesis of severe dengue. This study established an in-host model to characterize the plasma kinetics of dengue virus and NS1 with host immunity and evaluated the effects of ivermectin, using a population pharmacokinetic-pharmacodynamic (PK-PD) modeling approach, based on two studies in acute dengue fever: a placebo-controlled ivermectin study in 250 adult patients and an ivermectin PK-PD study in 24 pediatric patients. The proposed model described adequately the observed ivermectin pharmacokinetics, viral load, and NS1 data. Bodyweight was a significant covariate on ivermectin pharmacokinetics. We found that ivermectin reduced NS1 with an EC50 of 67.5 μg/mL. In silico simulations suggested that ivermectin should be dosed within 48 h after fever onset, and that a daily dosage of 800 μg/kg could achieve substantial NS1 reduction. The in-host dengue model is useful to assess the drug effect in antiviral drug development for dengue fever.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
-
- Control ECfDPa . 2‐Month Dengue Virus Disease Case Notification Rate per 100 000 Population. November 2022–October 2023. 2023. https://www.ecdc.europa.eu/en/publications‐data/12‐month‐dengue‐virus‐di...
-
- World Health Organization . WHO Position Paper on Dengue. 2018.
-
- Tricou V, Yu D, Reynales H, et al. Long‐term efficacy and safety of a tetravalent dengue vaccine (TAK‐003): 4.5‐year results from a phase 3, randomised, double‐blind, placebo‐controlled trial. lancet glob . Health. 2024;12:e257‐e270. - PubMed
-
- WHO . Summary of WHO Position Paper on Dengue Vaccines (2018). World Health Organization; 2018.
-
- Hadinegoro SR, Arredondo‐García JL, Capeding MR, et al. Efficacy and long‐term safety of a dengue vaccine in regions of endemic disease. N Engl J Med. 2015;373:1195‐1206. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

