mRNA vaccines: a new era in vaccine development
- PMID: 39308511
- PMCID: PMC11413818
- DOI: 10.32604/or.2024.043987
mRNA vaccines: a new era in vaccine development
Abstract
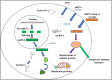
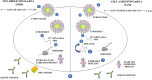
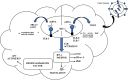
The advent of RNA therapy, particularly through the development of mRNA cancer vaccines, has ushered in a new era in the field of oncology. This article provides a concise overview of the key principles, recent advancements, and potential implications of mRNA cancer vaccines as a groundbreaking modality in cancer treatment. mRNA cancer vaccines represent a revolutionary approach to combatting cancer by leveraging the body's innate immune system. These vaccines are designed to deliver specific mRNA sequences encoding cancer-associated antigens, prompting the immune system to recognize and mount a targeted response against malignant cells. This personalized and adaptive nature of mRNA vaccines holds immense potential for addressing the heterogeneity of cancer and tailoring treatments to individual patients. Recent breakthroughs in the development of mRNA vaccines, exemplified by the success of COVID-19 vaccines, have accelerated their application in oncology. The mRNA platform's versatility allows for the rapid adaptation of vaccine candidates to various cancer types, presenting an agile and promising avenue for therapeutic intervention. Clinical trials of mRNA cancer vaccines have demonstrated encouraging results in terms of safety, immunogenicity, and efficacy. Pioneering candidates, such as BioNTech's BNT111 and Moderna's mRNA-4157, have exhibited promising outcomes in targeting melanoma and solid tumors, respectively. These successes underscore the potential of mRNA vaccines to elicit robust and durable anti-cancer immune responses. While the field holds great promise, challenges such as manufacturing complexities and cost considerations need to be addressed for widespread adoption. The development of scalable and cost-effective manufacturing processes, along with ongoing clinical research, will be pivotal in realizing the full potential of mRNA cancer vaccines. Overall, mRNA cancer vaccines represent a cutting-edge therapeutic approach that holds the promise of transforming cancer treatment. As research progresses, addressing challenges and refining manufacturing processes will be crucial in advancing these vaccines from clinical trials to mainstream oncology practice, offering new hope for patients in the fight against cancer.
Keywords: Cancer immunotherapy; Delivery system; Immune checkpoint; Preventive & therapeutic vaccine; mRNA.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no conflicts of interest to report regarding the present study.
Figures

Similar articles
-
mRNA-based cancer vaccines: A novel approach to melanoma treatment.Adv Immunol. 2025;165:117-162. doi: 10.1016/bs.ai.2024.10.010. Epub 2024 Dec 3. Adv Immunol. 2025. PMID: 40449972 Review.
-
Emerging prospects of mRNA cancer vaccines: mechanisms, formulations, and challenges in cancer immunotherapy.Front Immunol. 2024 Nov 25;15:1448489. doi: 10.3389/fimmu.2024.1448489. eCollection 2024. Front Immunol. 2024. PMID: 39654897 Free PMC article. Review.
-
mRNA vaccine for cancer immunotherapy.Mol Cancer. 2021 Feb 25;20(1):41. doi: 10.1186/s12943-021-01335-5. Mol Cancer. 2021. PMID: 33632261 Free PMC article. Review.
-
Cancer mRNA vaccines: clinical application progress and challenges.Cancer Lett. 2025 Aug 10;625:217752. doi: 10.1016/j.canlet.2025.217752. Epub 2025 Apr 28. Cancer Lett. 2025. PMID: 40306545 Review.
-
The Rapid Development and Early Success of Covid 19 Vaccines Have Raised Hopes for Accelerating the Cancer Treatment Mechanism.Arch Razi Inst. 2021 Mar;76(1):1-6. doi: 10.22092/ari.2021.353761.1612. Epub 2021 Mar 1. Arch Razi Inst. 2021. PMID: 33818952 Free PMC article.
Cited by
-
Lipid Nanoparticles for mRNA Delivery in Cancer Immunotherapy.AAPS J. 2025 Mar 18;27(3):66. doi: 10.1208/s12248-025-01051-8. AAPS J. 2025. PMID: 40102316 Review.
-
Engineering Useful Microbial Species for Pharmaceutical Applications.Microorganisms. 2025 Mar 5;13(3):599. doi: 10.3390/microorganisms13030599. Microorganisms. 2025. PMID: 40142492 Free PMC article. Review.
-
Cancer Vaccines: A Promising Therapeutic Strategy in Advanced Solid Tumors.Vaccines (Basel). 2025 May 30;13(6):591. doi: 10.3390/vaccines13060591. Vaccines (Basel). 2025. PMID: 40573922 Free PMC article. Review.
-
RNA therapeutics in cardiovascular medicine.Curr Opin Cardiol. 2025 May 1;40(3):139-149. doi: 10.1097/HCO.0000000000001210. Epub 2025 Feb 18. Curr Opin Cardiol. 2025. PMID: 39998478 Review.
-
Clinical Applications of the Molecular Landscape of Melanoma: Integration of Research into Diagnostic and Therapeutic Strategies.Cancers (Basel). 2025 Apr 24;17(9):1422. doi: 10.3390/cancers17091422. Cancers (Basel). 2025. PMID: 40361349 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
