Surface modification strategies to reinforce the soft tissue seal at transmucosal region of dental implants
- PMID: 39308548
- PMCID: PMC11415887
- DOI: 10.1016/j.bioactmat.2024.08.042
Surface modification strategies to reinforce the soft tissue seal at transmucosal region of dental implants
Abstract
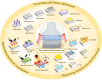
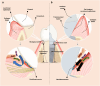
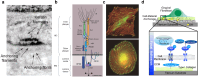
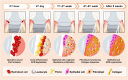
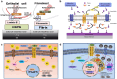
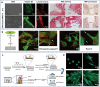
Soft tissue seal around the transmucosal region of dental implants is crucial for shielding oral bacterial invasion and guaranteeing the long-term functioning of implants. Compared with the robust periodontal tissue barrier around a natural tooth, the peri-implant mucosa presents a lower bonding efficiency to the transmucosal region of dental implants, due to physiological structural differences. As such, the weaker soft tissue seal around the transmucosal region can be easily broken by oral pathogens, which may stimulate serious inflammatory responses and lead to the development of peri-implant mucositis. Without timely treatment, the curable peri-implant mucositis would evolve into irreversible peri-implantitis, finally causing the failure of implantation. Herein, this review has summarized current surface modification strategies for the transmucosal region of dental implants with improved soft tissue bonding capacities (e.g., improving surface wettability, fabricating micro/nano topographies, altering the surface chemical composition and constructing bioactive coatings). Furthermore, the surfaces with advanced soft tissue bonding abilities can be incorporated with antibacterial properties to prevent infections, and/or with immunomodulatory designs to facilitate the establishment of soft tissue seal. Finally, we proposed future research orientations for developing multifunctional surfaces, thus establishing a firm soft tissue seal at the transmucosal region and achieving the long-term predictability of dental implants.
Keywords: Antibacterial; Dental implant transmucosal region; Immunomodulation; Soft tissue seal; Surface modifications.
© 2024 The Authors.
Conflict of interest statement
Yufeng Zheng is an editor-in-chief for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. Dandan Xia and Chunming Wang are editorial board members for Bioactive Materials and were not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures







References
-
- Atsuta I., Ayukawa Y., Kondo R., Oshiro W., Matsuura Y., Furuhashi A., Tsukiyama Y., Koyano K. Soft tissue sealing around dental implants based on histological interpretation. J. Prosthodont. Res. 2016;60:3–11. - PubMed
-
- Guo T.Q., Gulati K., Arora H., Han P.P., Fournier B. Race to invade: understanding soft tissue integration at the transmucosal region of titanium dental implants. Dent. Mater. 2021;37:816–831. - PubMed
-
- Albrektsson T., Wennerberg A. On osseointegration in relation to implant surfaces. Clin. Implant Dent. Relat. Res. 2019;21:4–7. - PubMed
-
- Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., Chen S., Cochran D., Derks J., Figuero E., Hämmerle C.H.F., Heitz-Mayfield L.J.A., Guy H.B., Iacono V., Koo K.T., Lambert F., McCauley L., Quirynen M., Renvert S., Salvi G.E., Schwarz F., Tarnow D., Tomasi C., Wang H.L., Zitzmann N. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45:S286–S291. - PubMed
-
- Derks J., Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015;42:S158–S171. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

