Independent and joint associations of cardiometabolic multimorbidity and depression on cognitive function: findings from multi-regional cohorts and generalisation from community to clinic
- PMID: 39308753
- PMCID: PMC11416683
- DOI: 10.1016/j.lanwpc.2024.101198
Independent and joint associations of cardiometabolic multimorbidity and depression on cognitive function: findings from multi-regional cohorts and generalisation from community to clinic
Abstract
Background: Cardiometabolic multimorbidity (CMM) and depression are often co-occurring in older adults and associated with neurodegenerative outcomes. The present study aimed to estimate the independent and joint associations of CMM and depression on cognitive function in multi-regional cohorts, and to validate the generalizability of the findings in additional settings, including clinical.
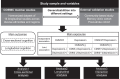
Methods: Data harmonization was performed across 14 longitudinal cohort studies within the Cohort Studies of Memory in an International Consortium (COSMIC) group, spanning North America, South America, Europe, Africa, Asia, and Australia. Three external validation studies with distinct settings were employed for generalization. Participants were eligible for inclusion if they had data for CMM and were free of dementia at baseline. Baseline CMM was defined as: 1) CMM 5, ≥2 among hypertension, hyperlipidemia, diabetes, stroke, and heart disease and 2) CMM 3 (aligned with previous studies), ≥2 among diabetes, stroke, and heart disease. Baseline depression was primarily characterized by binary classification of depressive symptom measurements, employing the Geriatric Depression Scale and the Center for Epidemiological Studies-Depression scale. Global cognition was standardized as z-scores through harmonizing multiple cognitive measures. Longitudinal cognition was calculated as changes in global cognitive z-scores. A pooled individual participant data (IPD) analysis was utilized to estimate the independent and joint associations of CMM and depression on cognitive outcomes in COSMIC studies, both cross-sectionally and longitudinally. Repeated analyses were performed in three external validation studies.
Findings: Of the 32,931 older adults in the 14 COSMIC cohorts, we included 30,382 participants with complete data on baseline CMM, depression, and cognitive assessments for cross-sectional analyses. Among them, 22,599 who had at least 1 follow-up cognitive assessment were included in the longitudinal analyses. The three external studies for validation had 1964 participants from 3 multi-ethnic Asian older adult cohorts in different settings (community-based, memory clinic, and post-stroke study). In COSMIC studies, each of CMM and depression was independently associated with cross-sectional and longitudinal cognitive function, without significant interactions between them (Ps > 0.05). Participants with both CMM and depression had lower cross-sectional cognitive performance (e.g. β = -0.207, 95% CI = (-0.255, -0.159) for CMM5 (+)/depression (+)) and a faster rate of cognitive decline (e.g. β = -0.040, 95% CI = (-0.047, -0.034) for CMM5 (+)/depression (+)), compared with those without either condition. These associations remained consistent after additional adjustment for APOE genotype and were robust in two-step random-effects IPD analyses. The findings regarding the joint association of CMM and depression on cognitive function were reproduced in the three external validation studies.
Interpretation: Our findings highlighted the importance of investigating age-related co-morbidities in a multi-dimensional perspective. Targeting both cardiometabolic and psychological conditions to prevent cognitive decline could enhance effectiveness.
Funding: Natural Science Foundation of China and National Institute on Aging/National Institutes of Health.
Keywords: Cardiometabolic multimorbidity; Cognitive decline; Depression; Multi-regional study.
© 2024 The Author(s).
Conflict of interest statement
CC declares funding to their institution from the National Medical Research Council of Singapore. RBL declares receiving payments for royalties or licenses from Wolff's Headache (7th and 8th Editions), Oxford University Press (2009), Wiley, and Inform. RBL has received consulting fees, honoraria for lectures, support for attending meetings and/or travel, or served on data safety monitoring boards or advisory boards for AbbVie (Allergan), the American Academy of Neurology, the American Headache Society, Amgen, Avanir, Axon, Axsome, Biohaven, Biovision, Boston Scientific, Dr. Reddy's (Promius), Electrocore, Eli Lilly, eNeura Therapeutics, Equinox, GlaxoSmithKline, Grifols, Lundbeck (Alder), Manistee, Merck, Pernix, Pfizer, Satsuma, Supernus, Teva, Trigemina, Vector, and Vedanta. RBL's institution has received funding from the FDA, the National Institutes of Health (NIH), and the National Institute on Aging (NIA). RBL holds stock in Axon, Biohaven Holdings, CoolTech, and Manistee. MJK declares funding to their institution from the NIH (grant numbers NIA P01 AG03949). NS declares participation as a member of the data safety monitoring board (uncompensated) for the public-private funded Phase II study PRimus AD in Germany and reports that their institution has received funding from Novo Nordisk, unrelated to the submitted work. Both HCH and SG declare funding to their institution from the NIH (grant numbers R01AG009956, P30AG072976, and K07AG076659). MG declares funding to their institution from the NIA (grant number R37AG023651). C–CHC declares that their institution received funding from the NIH. AL, CDC and EL declare that their institution received funding from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain (grant numbers 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128, 12/02254, 16/00896, 19/01874), as well as from the European Regional Development Fund (FEDER) of the European Union and the Government of Aragón (grant number B15_17R). CDC also declares receiving support for attending scientific meetings from Almirall, Lilly, Pfizer, Esteve, AstraZeneca, Novartis, Lundbeck, Casen Recordati, Janssen, and Rovi. PSS declares receiving payments for advisory board meetings for Biogen Australia and Roche Australia, honoraria for lectures from Alkem Labs; and funding to their institution from the National Health and Medical Research Council of Australia (grant number APP1169489). Both PSS and DL declare funding to their institution from the NIH (grant number 1RF1AG057531–01). Both PSS and JDC declare funding to their institution from the NIH (grant number 2R01AG057531-02A1). DL holds editor roles in the Frontiers journals. PSS holds leadership roles in the VASCOG Society (Executive Committee; unpaid) and the World Psychiatric Association (Planning Committee; unpaid). All other authors declare no competing interests.
Figures

References
-
- van Dyck C.H., Swanson C.J., Aisen P., et al. Lecanemab in early Alzheimer's disease. N Engl J Med. 2023;388:9–21. - PubMed
-
- Amoretti M., Amsler C., Bonomi G., et al. Production and detection of cold antihydrogen atoms. Nature. 2002;419:456–459. - PubMed
-
- Khondoker M., Macgregor A., Bachmann M.O., Hornberger M., Fox C., Shepstone L. Multimorbidity pattern and risk of dementia in later life: an 11-year follow-up study using a large community cohort and linked electronic health records. J Epidemiol Community Health. 2023;77:285–292. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

