Immunological signatures from irradiated cancer-associated fibroblasts
- PMID: 39308864
- PMCID: PMC11412886
- DOI: 10.3389/fimmu.2024.1433237
Immunological signatures from irradiated cancer-associated fibroblasts
Abstract
Introduction: Cancer-associated fibroblasts (CAFs) are abundant and influential elements of the tumor microenvironment (TME), giving support to tumor development in multiple ways. Among other mechanisms, CAFs are important regulators of immunological processes occurring in tumors. However, CAF-mediated tumor immunomodulation in the context of radiotherapy remains poorly understood. In this study, we explore effects of radiation on CAF-derived immunoregulatory signals to the TME.
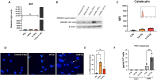
Methods: Primary CAF cultures were established from freshly collected human NSCLC lung tumors. CAFs were exposed to single-high or fractionated radiation regimens (1x18Gy or 3x6Gy), and the expression of different immunoregulatory cell-associated and secreted signaling molecules was analyzed 48h and 6 days after initiation of treatment. Analyses included quantitative measurements of released damage-associated molecular patterns (DAMPs), interferon (IFN) type I responses, expression of immune regulatory receptors, and secretion of soluble cytokines, chemokines, and growth factors. CAFs are able to survive ablative radiation regimens, however they enter into a stage of premature cell senescence.
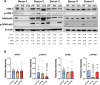
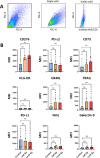
Results: Our data show that CAFs avoid apoptosis and do not contribute by release of DAMPs or IFN-I secretion to radiation-mediated tumor immunoregulation. Furthermore, the secretion of relevant immunoregulatory cytokines and growth factors including TGF-β, IL-6, IL-10, TNFα, IL-1β, VEGF, CXCL12, and CXCL10 remain comparable between non-irradiated and radiation-induced senescent CAFs. Importantly, radiation exposure modifies the cell surface expression of some key immunoregulatory receptors, including upregulation of CD73 and CD276.
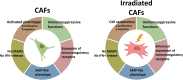
Discussion: Our data suggest that CAFs do not participate in the release of danger signals or IFN-I secretion following radiotherapy. The immune phenotype of CAFs and radiation-induced senescent CAFs is similar, however, the observed elevation of some cell surface immunological receptors on irradiated CAFs could contribute to the establishment of an enhanced immunosuppressive TME after radiotherapy.
Keywords: CAFs; NSCLC; cancer-associated fibroblasts; immunosuppression; ionizing radiation; non-small cell lung cancer; radiotherapy; tumor microenvironment.
Copyright © 2024 Berzaghi, Gundersen, Dille Pedersen, Utne, Yang, Hellevik and Martinez-Zubiaurre.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

