Implementing a new HCV model of care for people who use drugs
- PMID: 39308984
- PMCID: PMC11416665
- DOI: 10.1016/j.jhepr.2024.101145
Implementing a new HCV model of care for people who use drugs
Abstract
Background & aims: An estimated 50 million individuals have chronic hepatitis C virus (HCV) infection worldwide and people who use drugs (PWUD) are disproportionately affected. Persistent stigma and discrimination make it challenging for PWUD to access healthcare, potentially hindering HCV elimination progress in this population. To mitigate healthcare access barriers in PWUD, an HCV care model that simplified screening and linkage to care pathways was developed and rolled out in the Balearic Islands, Spain.
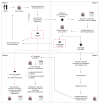
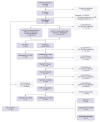
Methods: The prospective multicentre community model of care was implemented in 21 centres serving PWUD. This model involved: (1) participant recruitment and HCV antibody screening onsite via a point-of-care anti-HCV test, phlebotomy, or laboratory records; (2) HCV RNA, HBsAg and anti-HIV testing via a dried blood spot or phlebotomy; (3) linkage to specialist care and treatment prescription via telemedicine, when required; and (4) onsite monitoring of: (a) sustained virologic response (SVR) 4 and ≥12 weeks after treatment completion and; (b) potential new HCV infection or reinfection ∼1 year after phase 1 or SVR ≥12 monitoring. Care model acceptability was assessed.
Results: Between April 2021 and April 2023, 1,423 participants were recruited, of whom 464 (33%) were anti-HCV+ and 170 (12%) had detectable HCV RNA. Of the latter, 147 (86%) initiated therapy, of whom 124 (84%) completed it. SVR ≥12 monitoring was performed in 95 (77%) of these, of whom 88 (93%) had undetectable HCV RNA. Upon re-screening, four HCV reinfections were detected. Over 90% accepted study participation and screening and treatment decentralisation.
Conclusions: This adapted care model, which decentralised screening, diagnosis, and treatment, effectively increased healthcare access among PWUD, improving progress towards HCV elimination in this population in Spain.
Impact and implications: People who use drugs (PWUD) are among the most affected by chronic hepatitis C virus (HCV) infection globally. A simplified model of care was implemented in 21 centres serving this population across the Balearic Islands, Spain, to offer HCV care to 1,423 PWUD in 2021-2023. This decentralised screening, diagnosis, and treatment model resulted in an HCV cure rate of 93% of those who both completed therapy and were monitored post treatment completion. The Hepatitis C Free Balears model can guide the HCV elimination efforts of regional health authorities and other stakeholders in the rest of Spain and other parts of the world.
Keywords: Balearic Islands; Hepatitis C virus; Marginalised population; Micro-elimination; Point-of-care testing; Spain; Telemedicine; Viral hepatitis.
© 2024 The Author(s).
Figures



References
-
- World Health Organization. Hepatitis C: key facts, 2024. Available: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (Accessed 15 April 2024).
-
- World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030, 2022. https://www.who.int/publications/i/item/9789240053779 (Accessed 19 June 2023).
-
- World Health Organization. Global health sector strategy on viral hepatitis 2016-2021. Towards ending viral hepatitis, 2021. https://www.who.int/publications/i/item/WHO-HIV-2016.06 (Accessed 19 June 2023).
-
- Maucort-Boulch D., de Martel C., Franceschi S., et al. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–2477. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

