Hepatic estrogen receptor alpha drives masculinization in post-menopausal women with metabolic dysfunction-associated steatotic liver disease
- PMID: 39308985
- PMCID: PMC11414671
- DOI: 10.1016/j.jhepr.2024.101143
Hepatic estrogen receptor alpha drives masculinization in post-menopausal women with metabolic dysfunction-associated steatotic liver disease
Abstract
Background & aims: The loss of ovarian functions defining menopause leads to profound metabolic changes and heightens the risk of developing metabolic dysfunction-associated steatotic liver disease (MASLD). Although estrogens primarily act on the female liver through estrogen receptor alpha (ERα), the specific contribution of impaired ERα signaling in triggering MASLD after menopause remains unclear.
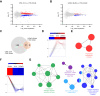
Methods: To address this gap in knowledge, we compared the liver transcriptomes of sham-operated (SHAM) and ovariectomized (OVX) control and liver ERα knockout (LERKO) female mice by performing RNA-Seq analysis.
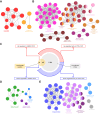
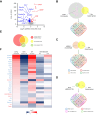
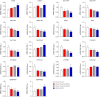
Results: OVX led to 1426 differentially expressed genes (DEGs) in the liver of control mice compared to 245 DEGs in LERKO mice. Gene ontology analysis revealed a distinct ovariectomy-induced modulation of the liver transcriptome in LERKO compared with controls, indicating that hepatic ERα is functional and necessary for the complete reprogramming of liver metabolism in response to estrogen depletion. Additionally, we observed an ovariectomy-dependent induction of male-biased genes, especially in the liver of control females, pointing to hepatic ERα involvement in the masculinization of the liver after estrogen loss. To investigate the translational relevance of such findings, we assessed liver samples from a cohort of 60 severely obese individuals (51 women; 9 men). Notably, a shift of the liver transcriptome toward a male-like profile was also observed only in obese women with MASLD (n = 43), especially in women ≥51 years old (15/15), suggesting that masculinization of the female liver contributes to MASLD development in obese women.
Conclusions: These results highlight the role of hepatic ERα in driving masculinization of the liver transcriptome following menopause, pointing to this receptor as a potential pharmacological target for preventing MASLD in post-menopausal women.
Impact and implications: Despite the increased risk of developing MASLD after menopause, the specific contribution of impaired hepatic estrogen signaling in driving MASLD in females has not been a major research focus, and, thus, has limited the development of tailored strategies that address the specific mechanisms underlying MASLD in post-menopausal women. This study reveals the functional role of hepatic ERα in mediating liver metabolic changes in response to estrogens loss, leading to a shift in the liver transcriptome towards a male-like profile. In women with obesity, this shift is associated with the development of MASLD. These findings underscore the potential of targeting hepatic ERα as a promising approach for developing effective, sex-specific treatments to preserve liver health and prevent or limit the development and progression of MASLD in post-menopausal women.
Keywords: Estrogens; Female; Liver metabolic programming; Menopause.
© 2024 The Authors.
Figures


References
-
- Powell E.E., Wong V.W.-S., Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–2224. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

