Exploring pharmacokinetic variability of palbociclib in HR+/HER2- metastatic breast cancer: a focus on age, renal function, and drug-gene interactions
- PMID: 39309010
- PMCID: PMC11412846
- DOI: 10.3389/fphar.2024.1420174
Exploring pharmacokinetic variability of palbociclib in HR+/HER2- metastatic breast cancer: a focus on age, renal function, and drug-gene interactions
Abstract
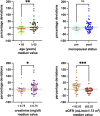
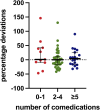
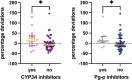
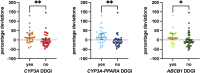
Palbociclib, an oral inhibitor of cyclin-dependent kinase 4 and 6, is approved for the treatment of metastatic breast cancer. This study investigated the influence of diverse clinical and biological factors-age, renal function, genetic variations, and concomitant medications (pharmacokinetic covariates)-on palbociclib pharmacokinetics. Employing a validated LC-MS/MS method, we analyzed the minimum plasma concentrations (Ctrough) of palbociclib in 68 women and determined the percentage deviations from the median Ctrough for each dosage group. Variations in a panel of absorption, distribution, metabolism, and excretion (ADME) genes were assessed using end-point allele-specific fluorescence detection and pyrosequencing. Two distinct patient cohorts were defined based on median values of age, creatinine, and eGFR, which exhibited statistically significant differences in percentage deviations (p = 0.0095, p = 0.0288, and p = 0.0005, respectively). Homozygous carriers of the PPARA variants displayed larger positive percentage deviations than the other group (p = 0.0292). Similarly, patients concurrently taking CYP3A and P-glycoprotein inhibitors alongside anticancer therapy exhibited significant variations (p = 0.0285 and p = 0.0334, respectively). Furthermore, exploring the drug-drug-gene interactions between inhibitors of CYP3A and P-glycoprotein with their respective genetic variants revealed two patient groups with statistically different percentage deviations (p = 0.0075, p = 0.0012, and p = 0.0191, respectively). These results could help address cases where pharmacokinetic covariates or subclinical conditions impair palbociclib adherence or response, aiming to offer tailored dosing strategies or monitoring for individual patients.
Keywords: drug-drug interactions; drug-drug-gene interactions; drug-gene interaction; metastatic breast cancer; minimum plasma concentration; palbociclib; pharmacokinetic covariate; pharmacokinetic variability.
Copyright © 2024 Peruzzi, Posocco, Gerratana, Nuti, Orleni, Gagno, De Mattia, Puglisi, Cecchin, Toffoli and Roncato.
Conflict of interest statement
LG reports consulting or advisory roles with AstraZeneca, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Incyte, Novartis, Pfizer, Menarini Stemmline, and AbbVie; and research funding for Menarini Silicon Biosystems. FP reports consulting or advisory roles with Roche, MSD, AstraZeneca, Novartis, Eli Lilly, Pfizer, Pierre Fabre, Daiichi Sankyo, Eisai, and Amgen, in addition to research funding from Eisai, AstraZeneca, and Roche. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
-
- Beck G. (2023). Modification of diet in renal Disease. 97821915 MB. 10.58020/H754-EG50 - DOI
-
- Bonotto M., Gerratana L., Di Maio M., De Angelis C., Cinausero M., Moroso S., et al. (2017). Chemotherapy versus endocrine therapy as first-line treatment in patients with luminal-like HER2-negative metastatic breast cancer: a propensity score analysis. Breast 31, 114–120. 10.1016/j.breast.2016.10.021 - DOI - PubMed
-
- Center for Drug Evaluation and Research (2014). Clinical pharmacology and biopharmaceutics review. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207103orig1s000c... (Accessed February 10, 2024).
-
- Clinical Pharmacology School of Medicine (2024). Cytochrome P450 Drug Interaction Table TM . Available at: https://drug-interactions.medicine.iu.edu/MainTable.aspx (Accessed February 10, 2024).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

