Suspected adverse drug reactions of rivaroxaban reported in the United States food and drug administration adverse event reporting system database: a pharmacovigilance study
- PMID: 39309013
- PMCID: PMC11412890
- DOI: 10.3389/fphar.2024.1399172
Suspected adverse drug reactions of rivaroxaban reported in the United States food and drug administration adverse event reporting system database: a pharmacovigilance study
Abstract
Purpose: This study aimed to characterize the safety profiles of rivaroxaban-associated suspected adverse events by mining the Food and Drug Administration Adverse Event Reporting System (FAERS).
Methods: A disproportionality analysis of spontaneously reported suspected adverse drug reactions (ADRs) was conducted. The reports in FAERS from 2014 to 2024 were compiled. Frequentist and Bayesian statistics were both applied to calculate drug-AE combinations in system organ classes and preferred-term levels. Reporting odds ratio (ROR), proportional reporting ratio (PRR), the Medicines and Healthcare products Regulatory Agency (MHRA), Bayesian confidence propagation neural network (BCPNN), and multi-item gamma Poisson shrinker (MGPS) methods were analyzed and used to compare the suspected AEs.
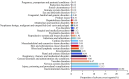
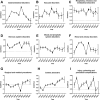
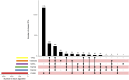
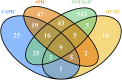
Results: Of 77,384 ADR reports, 66,705 (86.20%) were serious rivaroxaban AE reports. The most common age group was above 65 years. The suspected adverse effects of rivaroxaban emerging for system organ classes (SOCs) primarily included "Gastrointestinal disorders"; "Injury, poisoning, and procedural complications", "Nervous system disorders" and "Vascular disorders". Ranked by EBGM, the top signal strength of suspected AE signals of rivaroxaban under ROR algorithm at the preferred-term (PT) level were "Haemorrhagic arteriovenous malformation" (N = 571, ROR = 756.520, PRR = 754.029, Information Component (IC) = 7.197, Empirical Bayesian Geometric Mean (EBGM) = 146.725), "Gastrointestinal vascular malformation haemorrhagic" (N = 197, ROR = 211.138, PRR = 210.950, IC = 6.614, EBGM = 97.923), and "Diverticulum intestinal haemorrhagic" (N = 722, ROR = 169.898, PRR = 169.210, IC = 6.458, EBGM = 97.920). Moreover, uncommon but significantly suspected AE signals, such as "Coagulation factor X level increased", "Basal ganglia haematoma", and "Proctitis haemorrhagic" were observed. Notably, "Gastrointestinal haemorrhage" (N = 13,436, ROR = 80.477, PRR = 74.460, IC = 5.729, EBGM = 53.042), "Upper gastrointestinal haemorrhage"(N = 2,872, ROR = 73.978, PRR = 72.797, IC = 5.706, EBGM = 52.198) and "Internal haemorrhage" (N = 2,368, ROR = 91.979, PRR = 80.899, IC = 5.813, EBGM = 56.212) exhibited relatively high occurrence rates and signal strengths. From 2014 to 2024, the IC values of rivaroxaban-associated suspected AEs for "Surgical and medical procedures" and "Cardiac disorders" showed an annual increasing trend in the time-span analysis. Based on the various visulization plots, a key discovery is that "Gastrointestinal hemorrhage" emerged as the most significant suspected AE across five algorithms. The exciting finding was that the MGPS algorithm revealed a higher risk of suspected AEs under the "Investigations" category. However, the results of the analyses of the other algorithms at the SOC level were not akin to this. Moreover, the results of signal mining for the three main types of indication populations with adverse drug reactions (ADRs), including Atrial fibrillation, Cerebrovascular accident prophylaxis, and Deep vein thrombosis were shown that "Gastrointestinal haemorrhage", "Epistaxis", "Haematuria", "Rectal haemorrhage", and "Upper gastrointestinal haemorrhage" were detected as the most common and significant signals of suspected adverse events.
Conclusion: Rivaroxaban has risks of various suspected adverse reactions while providing therapeutic effects and being used widely. Our pharmacovigilance study may provide valuable hints that practitioners should closely monitor occurrences of "Gastrointestinal disorders", "Injury, poisoning, and procedural complications" and "Nervous system disorders", and other events in clinical applications. Consequently, it remains to persist in monitoring rivaroxaban, assessing the associated risks in the future.
Keywords: disproportionality analysis; drug safety; pharmacovigilance; rivaroxaban; suspected adverse event.
Copyright © 2024 Wu, Wu, Tang, Wang, Wei, Zhang, Li, Li, Wang, Wu and Hong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- AG. BP (2016). Xarelto1 summary of product characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/xarelto-epar-... .
LinkOut - more resources
Full Text Sources
Research Materials

