Serum amyloid A contributes to radiation-induced lung injury by activating macrophages through FPR2/Rac1/NF-κB pathway
- PMID: 39309438
- PMCID: PMC11414394
- DOI: 10.7150/ijbs.100823
Serum amyloid A contributes to radiation-induced lung injury by activating macrophages through FPR2/Rac1/NF-κB pathway
Abstract
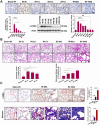
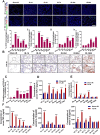
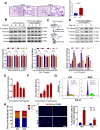
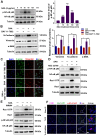
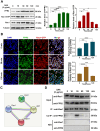
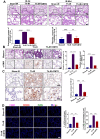
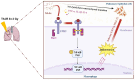
Patients who receive thoracic radiotherapy may suffer from radiation-induced lung injury, but the treatment options are limited as the underlying mechanisms are unclear. Using a mouse model of right thorax irradiation with fractionated doses of X-rays for three consecutive days (8 Gy/per day), this study found that the thoracic irradiation (Th-IR) induced tissue injury with aberrant infiltration of macrophages, and it significantly increased the secretion of TNF-α, IL-1β, IL-6, TGF-β1 and serum amyloid A (SAA) in mice. Interestingly, SAA could activate macrophages and then induce epithelial-mesenchymal transition (EMT) of lung epithelial cells and fibrosis progression in lung tissue. Mechanistically, SAA enhanced the transient binding of FPR2 to Rac1 protein and further activated NF-κB signaling pathway in macrophages. Inhibition of FPR2 significantly reduced pulmonary fibrosis induced by SAA administration in mice. In addition, cimetidine could reduce the level of SAA release after irradiation and attenuate the lung injury induced by SAA or Th-IR. In conclusion, our results demonstrated that SAA activated macrophages via FPR2/Rac1/NF-κB pathway and might contribute to the Th-IR induced lung injury, which may provide a new strategy to attenuate radiation-induced adverse effects during radiotherapy.
Keywords: FPR2 and NF-κB; Lung injury; Macrophages; SAA; Thoracic irradiation.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- Meattini I, Guenzi M, Fozza A. et al. Overview on cardiac, pulmonary and cutaneous toxicity in patients treated with adjuvant radiotherapy for breast cancer. Breast Cancer. 2017;24:52–62. - PubMed
-
- Ahmad SS, Duke S, Jena R. et al. Advances in radiotherapy. BMJ. 2012;345:e7765. - PubMed
-
- Turkkan G, Willems Y, Hendriks LEL. et al. Idiopathic pulmonary fibrosis: Current knowledge, future perspectives and its importance in radiation oncology. Radiother Oncol. 2021;155:269–77. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

