METTL18 functions as a Phenotypic Regulator in Src-Dependent Oncogenic Responses of HER2-Negative Breast Cancer
- PMID: 39309445
- PMCID: PMC11414398
- DOI: 10.7150/ijbs.96487
METTL18 functions as a Phenotypic Regulator in Src-Dependent Oncogenic Responses of HER2-Negative Breast Cancer
Abstract
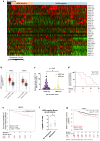
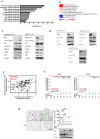
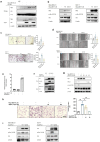
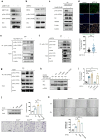
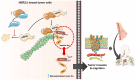
Methyltransferase-like (METTL)18 has histidine methyltransferase activity on the RPL3 protein and is involved in ribosome biosynthesis and translation elongations. Several studies have reported that actin polymerization serves as a Src regulator, and HSP90 is involved in forming polymerized actin bundles. To understand the role of METTL18 in breast cancer and to demonstrate the importance of METTL18 in HER-2 negative breast cancer metastasis, we used biochemical, molecular biological, and immunological approaches in vitro (breast tumor cell lines), in vivo (tumor xenograft model), and in samples of human breast tumors. A gene expression comparison of 31 METTL series genes and 22 methyltransferases in breast cancer patients revealed that METTL18 is highly amplified in human HER2-negative breast cancer. In addition, elevated levels of METTL18 expression in patients with HER2-negative breast cancer are associated with poor prognosis. Loss of METTL18 significantly reduced the metastatic responses of breast tumor cells in vitro and in vivo. Mechanistically, METTL18 indirectly regulates the phosphorylation of the proto-oncogene tyrosine-protein kinase Src and its downstream molecules in MDA-MB-231 cells via METTL18-mediated RPL3 methylation, which is also involved in determining HSP90 integrity and protein levels. In confocal microscopy and F/G-actin assays, METTL18 was found to induce actin polymerization via HSP90. Molecular events involving METTL18, RPL3, HSP90, and actin polymerization yielded Src phosphorylated at both tyrosine 419 and tyrosine 530 with kinase activity and oncogenic functions. Therefore, it is suggested that the METTL18-HSP90-Actin-Src regulatory axis plays critical oncogenic roles in the metastatic responses of HER2-negative breast cancer and could be a promising therapeutic target.
Keywords: HSP90; METTL18; RPL3 methylation; Src kinase; actin; breast cancer; histidine methyltransferase; invasion; metastasis; migration.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. - PubMed
-
- Vallejos CS, Gomez HL, Cruz WR, Pinto JA, Dyer RR, Velarde R. et al. Breast cancer classification according to immunohistochemistry markers: subtypes and association with clinicopathologic variables in a peruvian hospital database. Clin Breast Cancer. 2010;10:294–300. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

