Heme oxygenase 1-mediated ferroptosis in Kupffer cells initiates liver injury during heat stroke
- PMID: 39309491
- PMCID: PMC11413699
- DOI: 10.1016/j.apsb.2024.05.007
Heme oxygenase 1-mediated ferroptosis in Kupffer cells initiates liver injury during heat stroke
Abstract
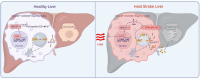
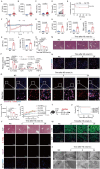
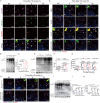
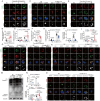
With the escalating prevalence of global heat waves, heat stroke has become a prominent health concern, leading to substantial liver damage. Unlike other forms of liver injury, heat stroke-induced damage is characterized by heat cytotoxicity and heightened inflammation, directly contributing to elevated mortality rates. While clinical assessments have identified elevated bilirubin levels as indicative of Kupffer cell dysfunction, their specific correlation with heat stroke liver injury remains unclear. Our hypothesis proposes the involvement of Kupffer cell ferroptosis during heat stroke, initiating IL-1β-mediated inflammation. Using single-cell RNA sequencing of murine macrophages, a distinct and highly susceptible Kupffer cell subtype, Clec4F+/CD206+, emerged, with heme oxygenase 1 (HMOX-1) playing a pivotal role. Mechanistically, heat-induced HMOX-1, regulated by early growth response factor 1, mediated ferroptosis in Kupffer cells, specifically in the Clec4F+/CD206+ subtype (KC2), activating phosphatidylinositol 4-kinase beta and promoting PI4P production. This cascade triggered NLRP3 inflammasome activation and maturation of IL-1β. These findings underscore the critical role of targeted therapy against HMOX-1 in ferroptosis within Kupffer cells, particularly in Clec4F+/CD206+ KCs. Such an approach has the potential to mitigate inflammation and alleviate acute liver injury in the context of heat stroke, offering a promising avenue for future therapeutic interventions.
Keywords: Early growth response factor 1; Ferroptosis; Heat stroke; Heme oxygenase 1; Kupffer cells; Liver injury; NLRP3; Phosphatidylinositol 4-kinase beta.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Bouchama A., Abuyassin B., Lehe C., Laitano O., Jay O., O'Connor F.G., et al. Classic and exertional heatstroke. Nat Rev Dis Primers. 2022;8:8. - PubMed
-
- Hassanein T., Razack A., Gavaler J.S., Van Thiel D.H. Heatstroke: its clinical and pathological presentation, with particular attention to the liver. Am J Gastroenterol. 1992;87:1382–1389. - PubMed
-
- Yezli S., Yassin Y., Ghallab S., Abdullah M., Abuyassin B., Vishwakarma R., et al. Classic heat stroke in a desert climate: a systematic review of 2632 cases. J Intern Med. 2023;294:7–20. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

