Kinetics of humoral and cellular immune responses 5 months post-COVID-19 booster dose by immune response groups at the peak immunity phase: An observational historical cohort study using the Fukushima vaccination community survey
- PMID: 39309610
- PMCID: PMC11416657
- DOI: 10.1016/j.jvacx.2024.100553
Kinetics of humoral and cellular immune responses 5 months post-COVID-19 booster dose by immune response groups at the peak immunity phase: An observational historical cohort study using the Fukushima vaccination community survey
Abstract
Background: Understanding the waning of immunity after booster vaccinations is important to identify which immune-low populations should be prioritized.
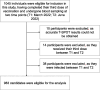
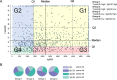
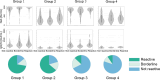
Methods: We investigated longitudinal cellular and humoral immunity after the third vaccine dose in both high- and low-cellular and humoral immunity groups at the peak immunity phase after the booster vaccination in a large community-based cohort. Blood samples were collected from 1045 participants at peak (T1: median 54 days post-third dose) and decay (T2: median 145 days post-third dose) phases to assess IgG(S), neutralizing activity, and ELISpot responses. Participants were categorized into high/low ELISpot/IgG(S) groups at T1. Cellular and humoral responses were tracked for approximately five months after the third vaccination.
Results: In total, 983 participants were included in the cohort. IgG(S) geometric mean fold change between timepoints revealed greater waning in the >79 years age group (T2/T1 fold change: 0.27) and higher IgG(S) fold change in the low-ELISpot group at T1 (T2/T1 fold change: 0.32-0.33) than in the other groups, although ELISpot geometric mean remained stable.
Conclusions: Antibody level of those who did not respond well to third dose vaccination waned rapidly than those who responded well. Evidence-based vaccine strategies are essential in preventing potential health issues caused by vaccines, including side-effects.
Keywords: Booster dose vaccine effectiveness; COVID-19; Cellular immunity; Humoral immunity; SARS-CoV-2.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kaneko is employed by Medical & Biological Laboratories, Co. (MBL, Tokyo, Japan). MBL imported the testing material used in this research. Kaneko participated in the testing process but was not involved in the research design and analysis. Kobashi and Tsubokura received a grant from Pfizer Health Research Foundation for research unrelated to this work.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

