36-Month Outcomes of Standalone Kahook Dual Blade Goniotomy Compared with Ab-Interno Closed Conjunctiva Xen Gel Stent Implantation
- PMID: 39309685
- PMCID: PMC11414631
- DOI: 10.2147/OPTH.S473303
36-Month Outcomes of Standalone Kahook Dual Blade Goniotomy Compared with Ab-Interno Closed Conjunctiva Xen Gel Stent Implantation
Abstract
Purpose: To compare the safety and effectiveness of standalone Kahook Dual Blade (KDB) excisional goniotomy to standalone ab-interno Xen gel stent implantation in eyes with moderate-to-severe open-angle glaucoma (OAG).
Methods: A single-center, retrospective study including eyes with moderate-to-severe OAG undergoing standalone KDB goniotomy or Xen gel stent implantation was conducted. Intraocular pressure (IOP), the number of antiglaucoma medications taken daily, and best-corrected visual acuity (BCVA) were recorded at baseline and for up to 36-months. Primary outcomes assessed included changes from baseline in IOP and the number of antiglaucoma medications taken. Intergroup comparisons were conducted using independent-samples Student's t-tests. The incidence of intraoperative and postoperative adverse events and the need for glaucoma surgical re-interventions were also recorded.
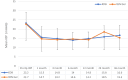
Results: Eyes receiving standalone KDB (n=26) or Xen gel stent (n=45) surgery were analyzed. The baseline mean IOP and number of antiglaucoma medications in both groups were as follows: KDB: 23.2 ± 6.0 mmHg, 2.2 ± 1.4 medications; Xen: 22.7 ± 8.8 mmHg, 3.0 ± 1.0 medications. At 36 months, IOP was reduced to 16.6 ± 5.4 mmHg in KDB eyes (n=23, -23.5%; p=0.0004) and 15.3 ± 5.6 mmHg in Xen gel stent eyes (n=15, -22.1%; p=0.006), while number of antiglaucoma medications was reduced to 1.1 ± 0.7 (-30.8%; p=0.0005) and 2.2 ± 1.4 (-25.6%; p=0.01), respectively. Three eyes (11.5%) in the KDB group and 19 eyes (42.2%) in the Xen gel stent group required additional surgery before month 36 due to refractory high IOP.
Conclusion: Both KDB goniotomy and Xen gel stent implantation significantly lowered the IOP and antiglaucoma medication burden in patients with moderate-to-severe OAG. While the Xen gel stent is frequently used to treat moderate-to-severe OAG patients with uncontrolled IOP, standalone KDB goniotomy may be equally effective as a long-term intervention, reducing the need for subsequent glaucoma surgery.
Keywords: kahook dual blade; micro-invasive glaucoma surgery; open angle glaucoma; xen gel stent.
© 2024 Boopathiraj et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures


References
-
- Van Veldhuisen PC, Ederer F, Gaasterland DE, et al. The advanced glaucoma intervention study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. - PubMed
LinkOut - more resources
Full Text Sources

