Bone sialoprotein stimulates cancer cell adhesion through the RGD motif and the αvβ3 and αvβ5 integrin receptors
- PMID: 39310027
- PMCID: PMC11413474
- DOI: 10.3892/ol.2024.14675
Bone sialoprotein stimulates cancer cell adhesion through the RGD motif and the αvβ3 and αvβ5 integrin receptors
Abstract
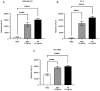
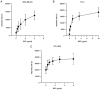
Being implicated in bone metastasis development, bone sialoprotein (BSP) expression is upregulated in patients with cancer. While BSP regulates cancer cell adhesion to the extracellular matrix, to the best of our knowledge, the specific adhesive molecular interactions in metastatic bone disease remain unclear. The present study aimed to improve the understanding of the arginine-glycine-aspartic acid (RGD) sequence of BSP and the integrin receptors αvβ3 and αvβ5 in BSP-mediated cancer cell adhesion. Human breast cancer (MDA-MB-231), prostate cancer (PC-3) and non-small cell lung cancer (NSCLC; NCI-H460) cell lines were cultured on BSP-coated plates. Adhesion assays with varying BSP concentrations were performed to evaluate the effect of exogenous glycine-arginine-glycine-aspartic acid-serine-proline (GRGDSP) peptide and anti-integrin antibodies on the attachment of cancer cells to BSP. Cell attachment was assessed using the alamarBlue® assay. The present results indicated that BSP supported the adhesion of cancer cells. The RGD counterpart GRGDSP peptide reduced the attachment of all tested cancer cell lines to BSP by ≤98.4%. Experiments with anti-integrin antibodies demonstrated differences among integrin receptors and cancer cell types. The αvβ5 antibody decreased NSCLC cell adhesion to BSP by 84.3%, while the αvβ3 antibody decreased adhesion by 14%. The αvβ3 antibody decreased PC-3 cell adhesion to BSP by 46.4%, while the αvβ5 antibody decreased adhesion by 9.5%. Adhesion of MDA-MB-231 cells to BSP was inhibited by 54.7% with αvβ5 antibody. The present results demonstrated that BSP-induced cancer cell adhesion occurs through the binding of the RGD sequence of BSP to the cell integrin receptors αvβ3 and αvβ5. Differences between cancer types were found regarding the mediation via αvβ3 or αvβ5 receptors. The present findings may explain why certain cancer cells preferentially spread to the bone tissue, suggesting that targeting the RGD-integrin binding interaction could be a promising novel cancer treatment option.
Keywords: BSP; RGD; cell adhesion; integrin receptors; metastasis.
Copyright: © 2024 Kottmann et al.
Conflict of interest statement
FPA is the CEO of Immundiagnostik AG and FC is an employee of Immundiagnostik AG. AB was an employee of Immundiagnostik AG at the time of the study. Immundiagnostik AG supplied the human bone sialoprotein and provided funding. The other authors declare that they have no competing interests.
Figures



Similar articles
-
Bone sialoprotein supports breast cancer cell adhesion proliferation and migration through differential usage of the alpha(v)beta3 and alpha(v)beta5 integrins.J Cell Physiol. 1998 Sep;176(3):482-94. doi: 10.1002/(SICI)1097-4652(199809)176:3<482::AID-JCP5>3.0.CO;2-K. J Cell Physiol. 1998. PMID: 9699501
-
Enamel matrix derivative is a potent inhibitor of breast cancer cell attachment to bone.Life Sci. 2005 Jan 28;76(11):1211-21. doi: 10.1016/j.lfs.2004.07.025. Life Sci. 2005. PMID: 15642592
-
Bone sialoprotein mediates human endothelial cell attachment and migration and promotes angiogenesis.Circ Res. 2000 Apr 28;86(8):885-91. doi: 10.1161/01.res.86.8.885. Circ Res. 2000. PMID: 10785511
-
Cy5.5-Knottin 2.5F.2009 Mar 26 [updated 2009 May 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Mar 26 [updated 2009 May 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641522 Free Books & Documents. Review.
-
Cy5.5-Knottin 2.5D.2009 Mar 26 [updated 2009 May 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2009 Mar 26 [updated 2009 May 28]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 20641277 Free Books & Documents. Review.
Cited by
-
In Situ Targeting RGD-Modified Cyclodextrin Inclusion Complex/Hydrogel Hybrid System for Enhanced Glioblastoma Therapy.Pharmaceutics. 2025 Jul 20;17(7):938. doi: 10.3390/pharmaceutics17070938. Pharmaceutics. 2025. PMID: 40733145 Free PMC article.
References
-
- Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;5:33588. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
