Is CONNECTDROP®, a Medication Event Monitoring System Add-On Paired with a Smartphone Application, Acceptable to Patients with Glaucoma for Taking Their Daily Medication? The CONDORE Pilot Study
- PMID: 39310045
- PMCID: PMC11416277
- DOI: 10.1016/j.xops.2024.100541
Is CONNECTDROP®, a Medication Event Monitoring System Add-On Paired with a Smartphone Application, Acceptable to Patients with Glaucoma for Taking Their Daily Medication? The CONDORE Pilot Study
Abstract
Objective: This pilot study tested the feasibility of a future efficacy trial examining the effect of CONNECTDROP®, a novel Medication Event Monitoring System (MEMS) paired with a mHealth application, on medication adherence in patients with glaucoma.
Design: A single-center, single-arm, prospective interventional pilot study (NCT04552964).
Participants: Adults with glaucoma managed with at least a fixed combination of timolol/dorzolamide who are adherent to treatment.
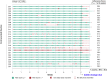
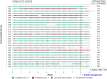
Methods: Participants (n = 31) were provided with the MEMS device and a smartphone with the application installed. They were required to use the MEMS with their usual timolol/dorzolamide prescription for 9 weeks. The study endpoint was at the end of week 9, when all study materials were returned, and participants completed a 17-item patient satisfaction questionnaire. Data collected continuously by each MEMS for the 9 weeks were analyzed for their suitability to quantify adherence of the individual participant and characterize adherence trends within the study cohort. Clinical data were collected at baseline, week 8, and week 9 for the safety evaluation.
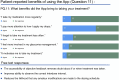
Main outcome measures: The primary outcome was global patient satisfaction after 9 weeks. Secondary outcome measures included participant feedback on handling the MEMS and its usability, along with that of the connected application. Objective data were used to determine participant medication adherence. The proportion of participants who successfully changed the MEMS to a new bottle at week 8 was reported.
Results: The MEMS-connected device achieved a global satisfaction score of 74.1% from study participants after 9 weeks. Furthermore, 70.4% of participants found the MEMS easy to use. However, only 59.2% reported feedback from the mHealth application useful in reminding them to take their treatment. MEMS-derived data showed that 70.4% of participants achieved an "adherence score" of 80% or above after 8 weeks and that 40.7% who completed the study had not changed the bottle correctly. No adverse events (AEs) were reported.
Conclusion: In this pilot study, the CONNECTDROP device was able to monitor daily intake of anti-glaucomatous medication over 2 months and had high satisfaction amongst this cohort of patients and was easy to use. The objective adherence data obtained appears reliable but must be validated for use in an efficacy trial.
Financial disclosures: The authors have no proprietary or commercial interest in any materials discussed in this article.
Keywords: Adherence; Glaucoma; MEMS; Medication Event Monitoring System; mHealth.
© 2024 Published by Elsevier Inc. on behalf of the American Academy of Ophthalmology.
Figures

 = Bluetooth wireless connection. MEMS = Medication Event Monitoring System.
= Bluetooth wireless connection. MEMS = Medication Event Monitoring System.

Similar articles
-
Accuracy of Patient-reported Adherence to Glaucoma Medications on a Visual Analog Scale Compared With Electronic Monitors.Clin Ther. 2015 Sep 1;37(9):1975-85. doi: 10.1016/j.clinthera.2015.06.008. Epub 2015 Jul 9. Clin Ther. 2015. PMID: 26164785 Free PMC article.
-
The utility of an electronic adherence assessment device in type 2 diabetes mellitus: a pilot study of single medication.Patient Prefer Adherence. 2010 Jul 21;4:247-54. doi: 10.2147/ppa.s10347. Patient Prefer Adherence. 2010. PMID: 20694184 Free PMC article.
-
Using an Electronic Medication Event-Monitoring System for Antiretroviral Therapy Self-Management Among African American Women Living With HIV in Rural Florida: Cohort Study.JMIR Form Res. 2020 Feb 19;4(2):e14888. doi: 10.2196/14888. JMIR Form Res. 2020. PMID: 32130114 Free PMC article.
-
Outcome measures for adherence data from a medication event monitoring system: A literature review.J Clin Pharm Ther. 2019 Feb;44(1):1-5. doi: 10.1111/jcpt.12757. Epub 2018 Sep 1. J Clin Pharm Ther. 2019. PMID: 30171815 Free PMC article. Review.
-
A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence.Br J Clin Pharmacol. 2016 Jul;82(1):268-79. doi: 10.1111/bcp.12942. Epub 2016 May 2. Br J Clin Pharmacol. 2016. PMID: 27005306 Free PMC article.
References
-
- Friedman D.S., Quigley H.A., Gelb L., et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS) Invest Ophthalmol Vis Sci. 2007;48:5052–5057. - PubMed
-
- Rossi G.C.M., Pasinetti G.M., Scudeller L., et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21:410–414. - PubMed
-
- Robin A.L., Muir K.W. Medication adherence in patients with ocular hypertension or glaucoma. Expert Rev Ophthalmol. 2019;14:199–210.
Associated data
LinkOut - more resources
Full Text Sources
Medical

