Pathophysiological dynamics in the contact, coagulation, and complement systems during sepsis: Potential targets for nafamostat mesilate
- PMID: 39310056
- PMCID: PMC11411436
- DOI: 10.1016/j.jointm.2024.02.003
Pathophysiological dynamics in the contact, coagulation, and complement systems during sepsis: Potential targets for nafamostat mesilate
Abstract
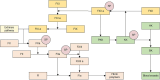
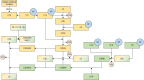
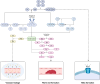
Sepsis is a life-threatening syndrome resulting from a dysregulated host response to infection. It is the primary cause of death in the intensive care unit, posing a substantial challenge to human health and medical resource allocation. The pathogenesis and pathophysiology of sepsis are complex. During its onset, pro-inflammatory and anti-inflammatory mechanisms engage in intricate interactions, possibly leading to hyperinflammation, immunosuppression, and long-term immune disease. Of all critical outcomes, hyperinflammation is the main cause of early death among patients with sepsis. Therefore, early suppression of hyperinflammation may improve the prognosis of these patients. Nafamostat mesilate is a serine protease inhibitor, which can inhibit the activation of the complement system, coagulation system, and contact system. In this review, we discuss the pathophysiological changes occurring in these systems during sepsis, and describe the possible targets of the serine protease inhibitor nafamostat mesilate in the treatment of this condition.
Keywords: Coagulation system; Complement system; Contact system; Nafamostat; Sepsis.
© 2024 The Authors.
Figures
Similar articles
-
Inhibition of enteral enzymes by enteroclysis with nafamostat mesilate reduces neutrophil activation and transfusion requirements after hemorrhagic shock.J Trauma. 2004 Mar;56(3):501-10; discussion 510-1. doi: 10.1097/01.ta.0000114536.98447.f7. J Trauma. 2004. PMID: 15128119
-
Nafamostat mesilate, as a treatment for heparin resistance, is not associated with perioperative ischemic stroke in patients undergoing cardiac surgery with cardiopulmonary bypass.J Cardiothorac Vasc Anesth. 2012 Apr;26(2):239-44. doi: 10.1053/j.jvca.2011.09.002. Epub 2011 Oct 22. J Cardiothorac Vasc Anesth. 2012. PMID: 22019206
-
Additional mechanisms of nafamostat mesilate-associated hyperkalaemia.Eur J Clin Pharmacol. 1996;51(2):149-51. doi: 10.1007/s002280050176. Eur J Clin Pharmacol. 1996. PMID: 8911880
-
Advances in the Study of Immunosuppressive Mechanisms in Sepsis.J Inflamm Res. 2023 Sep 8;16:3967-3981. doi: 10.2147/JIR.S426007. eCollection 2023. J Inflamm Res. 2023. PMID: 37706064 Free PMC article. Review.
-
Immunopathophysiology of human sepsis.EBioMedicine. 2022 Dec;86:104363. doi: 10.1016/j.ebiom.2022.104363. Epub 2022 Dec 2. EBioMedicine. 2022. PMID: 36470832 Free PMC article. Review.
Cited by
-
Small intestinal γδ T17 cells promote SAE through STING/C1q-induced microglial synaptic pruning in male mice.Nat Commun. 2025 Jul 23;16(1):6779. doi: 10.1038/s41467-025-62181-3. Nat Commun. 2025. PMID: 40702081 Free PMC article.
-
Common molecular profile of multiple structurally distinct warfare arsenicals in causing cutaneous chemical vesicant injury.Sci Rep. 2025 Feb 22;15(1):6505. doi: 10.1038/s41598-024-83513-1. Sci Rep. 2025. PMID: 39987158 Free PMC article.
-
Nafamostat mesylate augments survival in rats afflicted by exertional heat stroke.Front Pharmacol. 2025 May 15;16:1559181. doi: 10.3389/fphar.2025.1559181. eCollection 2025. Front Pharmacol. 2025. PMID: 40444038 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

