Lutetium-177-PSMA therapy for recurrent/metastatic salivary gland cancer: a prospective pilot study
- PMID: 39310108
- PMCID: PMC11413786
- DOI: 10.7150/thno.99035
Lutetium-177-PSMA therapy for recurrent/metastatic salivary gland cancer: a prospective pilot study
Abstract
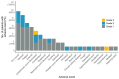
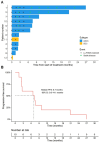
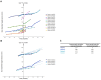
There is an urgent need for novel systemic therapies for recurrent/systemic salivary gland cancer, as current treatment options are scarce. [68Ga]Ga-PSMA-11 PET/CT revealed relevant uptake of prostate-specific membrane antigen (PSMA) in adenoid cystic carcinoma (AdCC) and salivary duct carcinoma (SDC). Therefore, we assessed the safety, feasibility, efficacy and radiation dosimetry of [177Lu]Lu-PSMA-I&T treatment in AdCC and SDC patients in a prospective pilot study. Methods: This single-center, single-arm study intended to include 10 recurrent/metastatic AdCC patients and five recurrent/metastatic SDC patients. AdCC patients could only participate in case of progressive and/or symptomatic disease. Patients required ≥ 1 lesion ≥ 1.5 cm with an SUVmax on [68Ga]Ga-PSMA-11 PET/CT above liver SUVmean. Patients were planned to receive four cycles ~ 7.4 GBq [177Lu]Lu-PSMA-I&T. In case of progressive disease per RECIST 1.1 at mid-treatment evaluation after two cycles, treatment was discontinued. Safety was the primary endpoint. Secondary endpoints included objective response rate (ORR), tumor- and organ-absorbed radiation doses and progression-free survival. Results: After screening, 10 out of 15 (67%) AdCC and two out of 10 (20%) SDC patients were eligible. Two patients (17%) demonstrated grade 3 treatment-related toxicity: lymphocytopenia (8%) and hyponatremia (8%). No dose-limiting toxicities occurred. In the AdCC cohort, six patients (60%) completed the four treatment cycles. Due to progressive disease, treatment was discontinued after two cycles in three patients (30%) and after one cycle in one patient (10%). No objective responses were observed (ORR: 0%). Three AdCC patients (30%) showed stable disease ≥ 6 months (7, 17 and 23 months). None of the two SDC patients completed the treatment: one patient deteriorated after the first cycle, while the other had progressive disease after two cycles. The high screen failure rate due to insufficient PSMA uptake resulted in premature closure of the SDC cohort. Dosimetry revealed low tumor-absorbed doses (median 0.07 Gy/GBq, range 0.001-0.63 Gy/GBq). Conclusions: [177Lu]Lu-PSMA-I&T in AdCC and SDC patients was safe and generally well-tolerated. However, efficacy was limited, likely due to low tumor-absorbed doses. For SDC, [177Lu]Lu-PSMA-I&T appears unfeasible due to insufficient PSMA uptake.
Keywords: (177)Lu-PSMA therapy; Adenoid cystic carcinoma; Prostate-specific membrane antigen; Salivary duct carcinoma; Salivary gland cancer.
© The author(s).
Conflict of interest statement
Competing Interests: NvR, MU, CD, SP, BP, AvEvG, MK, MK: nothing to declare. JN: has received research grants from ABX and Novartis, and has received consulting and teaching fees from ITM, POINT biopharma, CURIUM, Novartis and Bayer. CvH: has been member of advisory boards for Bayer, Bristol-Myers Squibb, Elevar, Ipsen, MSD and Regeneron, and has received research grants from Astra Zeneca, Bristol-Myers Squibb, MSD, Merck, Ipsen, Novartis and Sanofi.
Figures


References
-
- Spiro RH. Distant metastasis in adenoid cystic carcinoma of salivary origin. Am J Surg. 1997;174:495–8. - PubMed
-
- Ellington CL, Goodman M, Kono SA, Grist W, Wadsworth T, Chen AY. et al. Adenoid cystic carcinoma of the head and neck: Incidence and survival trends based on 1973-2007 Surveillance, Epidemiology, and End Results data. Cancer. 2012;118:4444–51. - PubMed
-
- van Weert S, Bloemena E, van der Waal I, de Bree R, Rietveld DH, Kuik JD, Leemans CR. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49:824–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

