The heterobivalent (SSTR2/albumin) radioligand [67Cu]Cu-NODAGA-cLAB4-TATE enables efficient somatostatin receptor radionuclide theranostics
- PMID: 39310112
- PMCID: PMC11413788
- DOI: 10.7150/thno.100091
The heterobivalent (SSTR2/albumin) radioligand [67Cu]Cu-NODAGA-cLAB4-TATE enables efficient somatostatin receptor radionuclide theranostics
Abstract
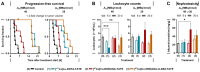
Somatostatin type 2 receptor (SSTR2) radionuclide therapy using β- particle-emitting radioligands has entered clinical practice for the treatment of neuroendocrine neoplasms (NENs). Despite the initial success of [177Lu]Lu‑DOTA-TATE, theranostic SSTR2 radioligands require improved pharmacokinetics and enhanced compatibility with alternative radionuclides. Consequently, this study evaluates the pharmacokinetic effects of the albumin-binding domain cLAB4 on theranostic performance of copper‑67-labeled NODAGA-TATE variants in an SSTR2-positive mouse pheochromocytoma (MPC) model. Methods: Binding, uptake, and release of radioligands as well as growth-inhibiting effects were characterized in cells grown as monolayers and spheroids. Tissue pharmacokinetics, absorbed tumor doses, and projected human organ doses were determined from quantitative SPECT imaging in a subcutaneous tumor allograft mouse model. Treatment effects on tumor growth, leukocyte numbers, and renal albumin excretion were assessed. Results: Both copper‑64- and copper‑67-labeled versions of NODAGA-TATE and NODAGA-cLAB4‑TATE showed similar SSTR2 binding affinity, but faster release from tumor cells compared to the clinical reference [177Lu]Lu‑DOTA-TATE. The bifunctional SSTR2/albumin-binding radioligand [67Cu]Cu‑NODAGA-cLAB4‑TATE showed both an improved uptake and prolonged residence time in tumors resulting in equivalent treatment efficacy to [177Lu]Lu‑DOTA-TATE. Absorbed doses were well tolerated in terms of leukocyte counts and kidney function. Conclusion: This preclinical study demonstrates therapeutic efficacy of [67Cu]Cu‑NODAGA-cLAB4‑TATE in SSTR2-positive tumors. As an intrinsic radionuclide theranostic agent, the radioligand provides stable radiocopper complexes and high sensitivity in SPECT imaging for prospective determination and monitoring of therapeutic doses in vivo. Beyond that, copper‑64- and copper‑61-labeled versions offer possibilities for pre- and post-therapeutic PET. Therefore, NODAGA-cLAB4-TATE has the potential to advance clinical use of radiocopper in SSTR2-targeted cancer theranostics.
Keywords: SPECT imaging; albumin binder; allograft model; copper-67; dosimetry; pheochromocytoma.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Basu S, Parghane RV, Kamaldeep, Chakrabarty S. Peptide receptor radionuclide therapy of neuroendocrine tumors. Semin Nucl Med. 2020;50:447–464. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

