The sperm-specific K+ channel Slo3 is inhibited by albumin and steroids contained in reproductive fluids
- PMID: 39310227
- PMCID: PMC11413451
- DOI: 10.3389/fcell.2024.1275116
The sperm-specific K+ channel Slo3 is inhibited by albumin and steroids contained in reproductive fluids
Abstract
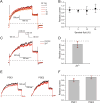
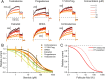
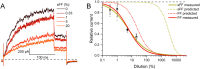
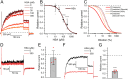
To locate and fertilize the egg, sperm probe the varying microenvironment prevailing at different stages during their journey across the female genital tract. To this end, they are equipped with a unique repertoire of mostly sperm-specific proteins. In particular, the flagellar Ca2+ channel CatSper has come into focus as a polymodal sensor used by human sperm to register ligands released into the female genital tract. Here, we provide the first comprehensive study on the pharmacology of the sperm-specific human Slo3 channel, shedding light on its modulation by reproductive fluids and their constituents. We show that seminal fluid and contained prostaglandins and Zn2+ do not affect the channel, whereas human Slo3 is inhibited in a non-genomic fashion by diverse steroids as well as by albumin, which are released into the oviduct along with the egg. This indicates that not only CatSper but also Slo3 harbours promiscuous ligand-binding sites that can accommodate structurally diverse molecules, suggesting that Slo3 is involved in chemosensory signalling in human sperm.
Keywords: follicular fluid; human sperm; ion channel; reproductive tract; sperm signalling.
Copyright © 2024 Lorenz, Eisenhardt, Mittermair, Kulle, Holterhus, Fobker, Boenigk, Nordhoff, Behre, Strünker and Brenker.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

