eIF2B localization and its regulation during the integrated stress response is cell-type specific
- PMID: 39310746
- PMCID: PMC11414691
- DOI: 10.1016/j.isci.2024.110851
eIF2B localization and its regulation during the integrated stress response is cell-type specific
Abstract
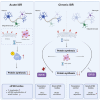
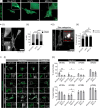
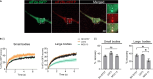
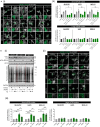
Eukaryotic initiation factor 2B (eIF2B) controls translation initiation by recycling inactive eIF2-GDP to active eIF2-GTP. Under cellular stress, the integrated stress response (ISR) is activated inhibiting eIF2B activity resulting in the translation attenuation and reprogramming of gene expression to overcome the stress. The ISR can dictate cell fate wherein chronic activation has pathological outcomes. Vanishing white matter disease (VWMD) is a chronic ISR-related disorder with mutations in eIF2B targeting astrocyte and oligodendrocyte cells. Regulation of eIF2B localization (eIF2B bodies) has been implicated in the ISR. We present evidence that neuronal and glial cell types possess distinct patterns of eIF2B bodies which change in a manner correlating to acute and chronic ISR activation. We also demonstrate that while neural and glial cell types respond similarly to the acute induction of the ISR a chronic ISR exerts cell-type specific differences. These findings provide key insights into neural cell responses and adaptation to cellular stress.
Keywords: Biological sciences; Cell; Molecular biology.
© 2024 The Author(s).
Conflict of interest statement
For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted article version of this article arising from this submission. The authors have no relevant financial or non-financial interests to disclose. The author Dr Filipe Hanson completed this work as part of his PhD studies at Sheffield Hallam University. He now works as a Research Scientist for UCB Pharma, Slough, UK. The author Dr Madalena deOliveira contributed to this work as part of her PhD studies at Sheffield Hallam University. She now works as a Postdoctoral Fellow for MSD, UK.
Figures



Similar articles
-
The small molecule ISRIB rescues the stability and activity of Vanishing White Matter Disease eIF2B mutant complexes.Elife. 2018 Feb 28;7:e32733. doi: 10.7554/eLife.32733. Elife. 2018. PMID: 29489452 Free PMC article.
-
Cellular eIF2B subunit localization: implications for the integrated stress response and its control by small molecule drugs.Mol Biol Cell. 2019 Apr 1;30(8):942-958. doi: 10.1091/mbc.E18-08-0538. Epub 2019 Feb 6. Mol Biol Cell. 2019. PMID: 30726166 Free PMC article.
-
Regulation and function of elF2B in neurological and metabolic disorders.Biosci Rep. 2022 Jun 30;42(6):BSR20211699. doi: 10.1042/BSR20211699. Biosci Rep. 2022. PMID: 35579296 Free PMC article. Review.
-
EIF2B2 mutations in vanishing white matter disease hypersuppress translation and delay recovery during the integrated stress response.RNA. 2018 Jun;24(6):841-852. doi: 10.1261/rna.066563.118. Epub 2018 Apr 9. RNA. 2018. PMID: 29632131 Free PMC article.
-
Regulation of translation initiation factor eIF2B at the hub of the integrated stress response.Wiley Interdiscip Rev RNA. 2018 Nov;9(6):e1491. doi: 10.1002/wrna.1491. Epub 2018 Jul 10. Wiley Interdiscip Rev RNA. 2018. PMID: 29989343 Review.
Cited by
-
Signaling plasticity in the integrated stress response.Front Cell Dev Biol. 2023 Dec 7;11:1271141. doi: 10.3389/fcell.2023.1271141. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38143923 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

