mCAUSE: Prioritizing mitochondrial targets that alleviate pancreatic cancer cell phenotypes
- PMID: 39310760
- PMCID: PMC11416656
- DOI: 10.1016/j.isci.2024.110880
mCAUSE: Prioritizing mitochondrial targets that alleviate pancreatic cancer cell phenotypes
Erratum in
-
Erratum: mCAUSE: Prioritizing mitochondrial targets that alleviate pancreatic cancer cell phenotypes.iScience. 2024 Oct 11;27(11):111146. doi: 10.1016/j.isci.2024.111146. eCollection 2024 Nov 15. iScience. 2024. PMID: 39759081 Free PMC article.
Abstract
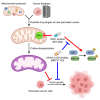
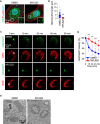
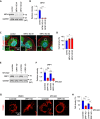
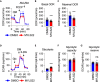
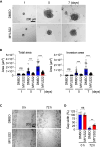
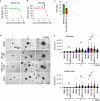
Substantial changes in energy metabolism are a hallmark of pancreatic cancer. To adapt to hypoxic and nutrient-deprived microenvironments, pancreatic cancer cells remodel their bioenergetics from oxidative phosphorylation to glycolysis. This bioenergetic shift makes mitochondria an Achilles' heel. Since mitochondrial function remains essential for pancreatic cancer cells, further depleting mitochondrial energy production is an appealing treatment target. However, identifying effective mitochondrial targets for treatment is challenging. Here, we developed an approach, mitochondria-targeted cancer analysis using survival and expression (mCAUSE), to prioritize target proteins from the entire mitochondrial proteome. Selected proteins were further tested for their impact on pancreatic cancer cell phenotypes. We discovered that targeting a dynamin-related GTPase, OPA1, which controls mitochondrial fusion and cristae, effectively suppresses pancreatic cancer activities. Remarkably, when combined with a mutation-specific KRAS inhibitor, OPA1 inhibition showed a synergistic effect. Our findings offer a therapeutic strategy against pancreatic cancer by simultaneously targeting mitochondria dynamics and KRAS signaling.
Keywords: Cancer; Cell biology; Molecular biology.
© 2024 The Author(s).
Conflict of interest statement
AJE has unlicensed patents related to keratin 14 as a prognostic marker and antibody strategy for anti-cancer therapeutics. AJE is a consultant for BioNTech. AJE’s spouse is an employee of ImmunoCore.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

