LRRK2G2019S Gene Mutation Causes Skeletal Muscle Impairment in Animal Model of Parkinson's Disease
- PMID: 39310961
- PMCID: PMC11634472
- DOI: 10.1002/jcsm.13604
LRRK2G2019S Gene Mutation Causes Skeletal Muscle Impairment in Animal Model of Parkinson's Disease
Abstract
Background: While the gradually aggravated motor and non-motor disorders of Parkinson's disease (PD) lead to progressive disability and frequent falling, skeletal muscle impairment may contribute to this condition. The leucine-rich repeat kinase2 (LRRK2) is a common disease-causing gene in PD. Little is known about its role in skeletal muscle impairment and its underlying mechanisms.
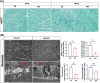
Methods: To investigate whether the mutation in LRRK2 causes skeletal muscle impairment, we used 3-month-old (3mo) and 14-month-old (14mo) LRRK2G2019S transgenic (TG) mice as a model of PD, compared with the age-matched littermate wild-type (WT) controls. We measured the muscle mass and strength, ultrastructure, inflammatory infiltration, mitochondrial morphology and dynamics dysfunction through behavioural analysis, electromyography (EMG), immunostaining, transmission electron microscopy (TEM) and other molecular biology techniques.
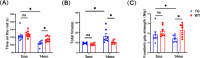
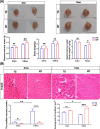
Results: The 3mo-TG mice display mild skeletal muscle impairment with spontaneous potentials in EMG (increased by 130%, p < 0.05), myofibre necrosis (p < 0.05) and myosin heavy chain-II changes (reduced by 19%, p < 0.01). The inflammatory cells and macrophage infiltration are significantly increased (CD8a+ and CD68+ cells up 1060% and 579%, respectively, both p < 0.0001) compared with the WT mice. All of the above pathogenic processes are aggravated by aging. The 14mo-TG mice EMG examinations show a reduced duration (by 31%, p < 0.01) and increased polyphasic waves of motor unit action potentials (by 28%, p < 0.05). The 14mo-TG mice present motor behavioural deficits (p < 0.05), muscle strength and mass reduction by 37% and 8% (p < 0.05 and p < 0.01, respectively). A remarkable increase in inflammatory infiltration is accompanied by pro-inflammatory cytokines in the skeletal muscles. TEM analysis shows muscle fibre regeneration with the reduced length of sarcomeres (by 6%;p < 0.05). The muscle regeneration is activated as Pax7+ cells increased by 106% (p < 0.0001), andmyoblast determination protein elevated by 71% (p < 0.01). We also document the morphological changes and dynamics dysfunction of mitochondria with the increase of mitofusin1 by 43% (p < 0.05) and voltage-dependent anion channel 1 by 115% (p < 0.001) in the skeletal muscles of 14mo-TG mice.
Conclusions: Taken together, these findings may provide new insights into the clinical and pathogenic involvement of LRRK2G2019 mutation in muscles, suggesting that the diseases may affect not only midbrain dopaminergic neurons, but also other tissues, and it may help overall clinical management of this devastating disease.
Keywords: LRRK2G2019S mutation; Parkinson's disease; electromyography; mitochondrial impairment; skeletal muscle impairment.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Samii A., Nutt J. G., and Ransom B. R., “Parkinson's Disease,” Lancet 363 (2004): 1783–1793. - PubMed
-
- Siciliano M., Trojano L., Santangelo G., De Micco R., Tedeschi G., and Tessitore A., “Fatigue in Parkinson's Disease: A Systematic Review and Meta‐Analysis,” Movement Disorders 33 (2018): 1712–1723. - PubMed
-
- Tinazzi M., Recchia S., Simonetto S., et al., “Muscular Pain in Parkinson's Disease and Nociceptive Processing Assessed With CO2 Laser‐Evoked Potentials,” Movement Disorders 25 (2010): 213–220. - PubMed
-
- Cruz‐Jentoft A. J. and Sayer A. A., “Sarcopenia,” Lancet 393 (2019): 2636–2646. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

