Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories
- PMID: 39311362
- PMCID: PMC11417764
- DOI: 10.3390/ijns10030060
Consistency in the Assessment of Dried Blood Spot Specimen Size and Quality in U.K. Newborn Screening Laboratories
Abstract
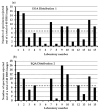
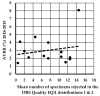
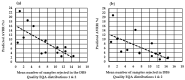
In 2015, U.K. newborn screening (NBS) laboratory guidelines were introduced to standardize dried blood spot (DBS) specimen quality acceptance and specify a minimum acceptable DBS diameter of ≥7 mm. The UK 'acceptable' avoidable repeat rate (AVRR) is ≤2%. To assess inter-laboratory variability in specimen acceptance/rejection, two sets of colored scanned images (n = 40/set) of both good and poor-quality DBS specimens were distributed to all 16 U.K. NBS laboratories for evaluation as part of an external quality assurance (EQA) assessment. The mean (range) number of specimens rejected in the first EQA distribution was 7 (1-16) and in the second EQA distribution was 7 (0-16), demonstrating that adherence to the 2015 guidelines was highly variable. A new minimum standard for DBS size of ≥8 mm (to enable a minimum of six sub-punches from two DBS) was discussed. NBS laboratories undertook a prospective audit and demonstrated that using ≥8 mm as the minimum acceptable DBS diameter would increase the AVRR from 2.1% (range 0.55% to 5.5%) to 7.8% (range 0.55% to 22.7%). A significant inverse association between the number of specimens rejected in the DBS EQA distributions and the predicted AVVR (using ≥8 mm minimum standard) was observed (r = -0.734, p = 0.003). Before implementing more stringent standards, the impact of a standard operating procedure (SOP) designed to enable a standardized approach of visual assessment and using the existing ≥7 mm diameter (to enable a minimum of four sub-punches from two DBS) as the minimum standard was assessed in a retrospective audit. Implementation of the SOP and using the ≥7 mm DBS diameter would increase the AVRR from 2.3% (range 0.63% to 5.3%) to 6.5% (range 4.3% to 20.9%). The results demonstrate that there is inconsistency in applying the acceptance/rejection criteria, and that a low AVVR is not an indication of good-quality specimens being received into laboratories. Further work is underway to introduce and maintain standards without increasing the AVRR to unacceptable levels.
Keywords: avoidable repeat rate; blood spot quality; dried blood spots; external quality assessment; filter paper; specimen collection; specimen collection device.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- U.K. Government Newborn Blood Spot Screening Data Collection and Performance Analysis Report 1 April 2018 to 31 March 2019. Newborn Blood Spot Screening Data Collection and Performance Analysis Report 1 April 2018 to 31 March 2019—GOV.UK. [(accessed on 27 January 2024)]; Available online: https://www.gov.uk.
-
- Moat S.J., Dibden C., Tetlow L., Griffith C., Chilcott J., George R., Hamilton L., Wu T.H., MacKenzie F., Hall S.K. Effect of blood volume on analytical bias in dried blood spots prepared for newborn screening external quality assurance. Bioanalysis. 2020;12:22–109. doi: 10.4155/bio-2019-0201. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

