Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century?
- PMID: 39311364
- PMCID: PMC11417796
- DOI: 10.3390/ijns10030062
Wilson and Jungner Revisited: Are Screening Criteria Fit for the 21st Century?
Abstract
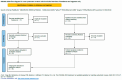
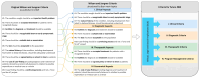
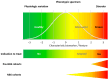
Driven by technological innovations, newborn screening (NBS) panels have been expanded and the development of genomic NBS pilot programs is rapidly progressing. Decisions on disease selection for NBS are still based on the Wilson and Jungner (WJ) criteria published in 1968. Despite this uniform reference, interpretation of the WJ criteria and actual disease selection for NBS programs are highly variable. A systematic literature search [PubMED search "Wilson" AND "Jungner"; last search 16.07.22] was performed to evaluate the applicability of the WJ criteria for current and future NBS programs and the need for adaptation. By at least two reviewers, 105 publications (systematic literature search, N = 77; manual search, N = 28) were screened for relevant content and, finally, 38 publications were evaluated. Limited by the study design of qualitative text analysis, no statistical evaluation was performed, but a structured collection of reported aspects of criticism and proposed improvements was instead collated. This revealed a set of general limitations of the WJ criteria, such as imprecise terminology, lack of measurability and objectivity, missing pediatric focus, and absent guidance on program management. Furthermore, it unraveled specific aspects of criticism on clinical, diagnostic, therapeutic, and economical aspects. A major obstacle was found to be the incompletely understood natural history and phenotypic diversity of rare diseases prior to NBS implementation, resulting in uncertainty about case definition, risk stratification, and indications for treatment. This gap could be closed through the systematic collection and evaluation of real-world evidence on the quality, safety, and (cost-)effectiveness of NBS, as well as the long-term benefits experienced by screened individuals. An integrated NBS public health program that is designed to continuously learn would fulfil these requirements, and a multi-dimensional framework for future NBS programs integrating medical, ethical, legal, and societal perspectives is overdue.
Keywords: genomic newborn screening; neonatal screening; newborn screening; newborn sequencing; phenotypic diversity; public health program; screening criteria.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
References
-
- Schulze A., Lindner M., Kohlmüller D., Olgemöller K., Mayatepek E., Hoffmann G.F. Expanded Newborn Screening for Inborn Errors of Metabolism by Electrospray Ionization-Tandem Mass Spectrometry: Results, Outcome, and Implications. Pediatrics. 2003;111:1399–1406. doi: 10.1542/peds.111.6.1399. - DOI - PubMed
-
- Watson M.S., Lloyd-Puryear M.A., Mann M.Y., Rinaldo P., Howell R., editors. Newborn Screening: Toward a Uniform Screening Panel and System. Genet. Med. 2006;8((Suppl. S1)):1s–252s. doi: 10.1097/01.gim.0000223891.82390.ad. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

