Functional Activity of Cytokine-Induced Killer Cells Enhanced by CAR-CD19 Modification or by Soluble Bispecific Antibody Blinatumomab
- PMID: 39311376
- PMCID: PMC11417890
- DOI: 10.3390/antib13030071
Functional Activity of Cytokine-Induced Killer Cells Enhanced by CAR-CD19 Modification or by Soluble Bispecific Antibody Blinatumomab
Abstract
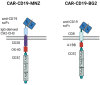
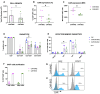
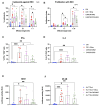
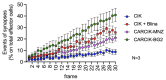
Strategies to increase the anti-tumor efficacy of cytokine-induced killer cells (CIKs) include genetic modification with chimeric antigen receptors (CARs) or the addition of soluble T-cell engaging bispecific antibodies (BsAbs). Here, CIKs were modified using a transposon system integrating two distinct anti-CD19 CARs (CAR-MNZ and CAR-BG2) or combined with soluble CD3xCD19 BsAb blinatumomab (CIK + Blina). CAR-MNZ bearing the CD28-OX40-CD3ζ signaling modules, and CAR-BG2, designed on the Tisagenlecleucel CAR sequence (Kymriah®), carrying the 4-1BB and CD3ζ signaling elements, were employed. After transfection and CIK expansion, cells expressed CAR-CD19 to a similar extent (35.9% CAR-MNZ and 17.7% CAR-BG2). In vitro evaluations demonstrated robust proliferation and cytotoxicity (~50% cytotoxicity) of CARCIK-MNZ, CARCIK-BG2, and CIK + Blina against CD19+ target cells, suggesting similar efficacy. All effectors formed an increased number of synapses, activated NFAT and NFkB, and secreted IL-2 and IFN-ɣ upon encountering targets. CIK + Blina displayed strongest NFAT and IFN-ɣ induction, whereas CARCIK-BG2 demonstrated superior synapse formation. All the effectors have shown therapeutic activity in vivo against the CD19+ Daudi tumor model, with CARCIK cells showing a more durable response compared to CIK + Blina, likely due to the short half-life of Blina in this model.
Keywords: B-cell neoplasia; CAR signaling; bispecific antibody; chimeric antigen receptor; cytokine-induced killer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Pievani A., Borleri G., Pende D., Moretta L., Rambaldi A., Golay J., Introna M. Dual-Functional Capability of CD3 + CD56 + CIK Cells, a T-Cell Subset That Acquires NK Function and Retains TCR-Mediated Specific Cytotoxicity. Blood. 2011;118:3301–3310. doi: 10.1182/blood-2011-02-336321. - DOI - PubMed
-
- Valgardsdottir R., Capitanio C., Texido G., Pende D., Cantoni C., Pesenti E., Rambaldi A., Golay J., Introna M. Direct Involvement of CD56 in Cytokine-Induced Killer-Mediated Lysis of CD56+ Hematopoietic Target Cells. Exp. Hematol. 2014;42:1013–1021.e1. doi: 10.1016/j.exphem.2014.08.005. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

