Long-Term Immunity against SARS-CoV-2 Wild-Type and Omicron XBB.1.5 in Indonesian Residents after Vaccination and Infection
- PMID: 39311377
- PMCID: PMC11417924
- DOI: 10.3390/antib13030072
Long-Term Immunity against SARS-CoV-2 Wild-Type and Omicron XBB.1.5 in Indonesian Residents after Vaccination and Infection
Abstract
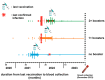
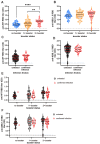
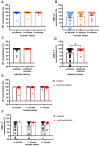
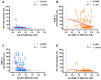
In the post-pandemic era, evaluating long-term immunity against COVID-19 has become increasingly critical, particularly in light of continuous SARS-CoV-2 mutations. This study aimed to assess the long-term humoral immune response in sera collected in Makassar. We measured anti-RBD IgG levels and neutralization capacity (NC) against both the Wild-Type (WT) Wuhan-Hu and Omicron XBB.1.5 variants across groups of COVID-19-vaccinated individuals with no booster (NB), single booster (SB), and double booster (DB). The mean durations since the last vaccination were 25.11 months, 19.24 months, and 16.9 months for the NB, SB, and DB group, respectively. Additionally, we evaluated the effect of breakthrough infection (BTI) history, with a mean duration since the last confirmed infection of 21.72 months. Our findings indicate fair long-term WT antibody (Ab) titers, with the DB group showing a significantly higher level than the other groups. Similarly, the DB group demonstrated the highest anti-Omicron XBB.1.5 Ab titer, yet it was insignificantly different from the other groups. Although the level of anti-WT Ab titers was moderate, we observed near-complete (96-97%) long-term neutralization against the WT pseudo-virus for all groups. There was a slight decrease in NC against Omicron XBB.1.5 compared to the WT among all groups, as DB group, SB group, and NB group showed 80.71 ± 3.9%, 74.29 ± 6.7%, and 67.2 ± 6.3% neutralization activity, respectively. A breakdown analysis based on infection and vaccine status showed that booster doses increase the NC against XBB.1.5, particularly in individuals without BTI. Individuals with BTI demonstrate a better NC compared to their counterpart uninfected individuals with the same number of booster doses. Our findings suggest that long-term immunity against SARS-CoV-2 persists and is effective against the mutant variant. Booster doses enhance the NC, especially among uninfected individuals.
Keywords: COVID-19 vaccines; Omicron XBB.1.5; antibodies; immune persistence; neutralizing capacity.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Distinct features of SARS-CoV-2 humoral immunity against Omicron breakthrough infection.Vaccine. 2023 Nov 13;41(47):7019-7025. doi: 10.1016/j.vaccine.2023.10.035. Epub 2023 Oct 17. Vaccine. 2023. PMID: 37858449
-
Protective immunity of SARS-CoV-2 infection and vaccines against medically attended symptomatic omicron BA.4, BA.5, and XBB reinfections in Singapore: a national cohort study.Lancet Infect Dis. 2023 Jul;23(7):799-805. doi: 10.1016/S1473-3099(23)00060-9. Epub 2023 Mar 13. Lancet Infect Dis. 2023. PMID: 36924786 Free PMC article.
-
SARS-CoV-2 Neutralization Capacity in Hemodialysis Patients with and without a Fifth Vaccination with the Updated Comirnaty Original/Omicron BA.4-5 Vaccine.Vaccines (Basel). 2024 Mar 15;12(3):308. doi: 10.3390/vaccines12030308. Vaccines (Basel). 2024. PMID: 38543942 Free PMC article.
-
Ad5-nCoV booster and Omicron variant breakthrough infection following two doses of inactivated vaccine elicit comparable antibody levels against Omicron variants.J Med Virol. 2023 Jan;95(1):e28163. doi: 10.1002/jmv.28163. Epub 2022 Oct 1. J Med Virol. 2023. PMID: 36127294 Free PMC article.
-
Kinetics of SARS-CoV-2 antibody titers after booster vaccinations during an Omicron surge in Japan.Vaccine. 2024 Aug 30;42(21):126156. doi: 10.1016/j.vaccine.2024.126156. Epub 2024 Jul 31. Vaccine. 2024. PMID: 39088986
References
-
- World Health Organization Statement on the Fifteenth Meeting of the IHR (2005) Emergency Committee on the COVID-19 Pandemic. [(accessed on 13 March 2024)]. Available online: https://www.who.int/news/item/05-05-2023-statement-on-the-fifteenth-meet....
-
- COVID-19 Epidemiological Update. Dec 22, 2023. [(accessed on 13 March 2024)]. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update-....
-
- Lau E.H.Y., Hui D.S.C., Tsang O.T.Y., Chan W.-H., Kwan M.Y.W., Chiu S.S., Cheng S.M.S., Ko R.L.W., Li J.K.C., Chaothai S., et al. Long-term persistence of SARS-CoV-2 neutralizing antibody responses after infection and estimates of the duration of protection. eClinicalMedicine. 2021;41:101174. doi: 10.1016/j.eclinm.2021.101174. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

