Results From a Psychometric Validation Study: Patients With Irritable Bowel Syndrome Report Higher Symptom Burden Using End-of-Day Vs Real-Time Assessment
- PMID: 39311432
- PMCID: PMC12043265
- DOI: 10.14309/ajg.0000000000003091
Results From a Psychometric Validation Study: Patients With Irritable Bowel Syndrome Report Higher Symptom Burden Using End-of-Day Vs Real-Time Assessment
Abstract
Introduction: Real-time assessment of gastrointestinal (GI) symptoms in irritable bowel syndrome (IBS) using the experience sampling method (ESM) is suggested as a more appropriate approach than currently used end-of-day or end-of-week reports. This psychometric evaluation study assesses the validity and reliability of a previously developed ESM-based patient-reported outcome measure (PROM) for real-time GI symptom assessment in IBS.
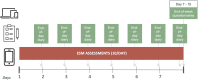
Methods: This multicenter validation study included 230 Rome IV patients with IBS (80% female; mean age 41.2 years) in 3 European countries. Patients completed the electronic ESM-PROM (up to 10 random moments daily, with a weekly minimum completion rate of 33%) and an end-of-day symptom diary for 7 consecutive days. End-of-week questionnaires (Gastrointestinal Symptom Rating Scale for IBS, IBS Severity Scoring System, Patient Health Questionnaire-9, and Generalized Anxiety Disorder-7) were completed at the end of the 7-day period.
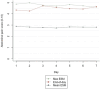
Results: The ESM assessment had a mean completion rate of 71%. Strong and significant correlations (0.651-0.956) with moderate-to-good consistency (intra-class correlation coefficients 0.580-0.779) were observed between ESM and end-of-day scores. However, end-of-day scores were significantly higher (Δ0.790-1.758, P < 0.001) than mean daily ESM scores. Differences with end-of-week scores were more pronounced, with weaker correlations (Pearson's r 0.393-0.802). ESM-PROM exhibited moderate-to-good internal consistency (Cronbach's α 0.585-0.887) across 5 symptom domains. First and second half-week scores demonstrated good-to-excellent consistency (intraclass correlation coefficients 0.871-0.958).
Discussion: Psychometric evaluation demonstrated strong validity and reliability of the ESM-PROM for real-time GI symptom assessment in IBS. In addition, the ESM-PROM provides a precise and reliable ascertainment of individual symptom pattern and trigger interactions, without the bias of peak reporting when compared with retrospective methods. This highlights its potential as a valuable tool for personalized healthcare in monitoring disease course and treatment response in patients with IBS.
Keywords: experience sampling method; irritable bowel syndrome; patient-reported outcome; psychometric evaluation.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures


References
-
- Lacy BE, Mearin F, Chang L, et al. . Bowel disorders. Gastroenterology 2016;150(6):1393–407.e5. - PubMed
-
- Mearin F, Lacy BE. Diagnostic criteria in IBS: Useful or not? Neurogastroenterol Motil 2012;24(9):791–801. - PubMed
-
- Tack J, Carbone F, Chang L, et al. . Patient reported outcomes in disorders of gut-brain interaction. Gastroenterology 2024;166(4):572–87.e1. - PubMed
-
- U.S. Department of Health and Human Services FDA. Guidance for industry irritable bowel syndrome–clinical evaluation of drugs for treatment. U.S. Food and Drug Administration: Silver Spring, MD, 2012.
-
- European Medicines Agency (EMA). Guideline on the Evaluation of Medicinal Products for the Treatment of Irritable Bowel Syndrome. EMA: London, UK, 2013.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

