REAL AD-Validation of a realistic screening approach for early Alzheimer's disease
- PMID: 39311530
- PMCID: PMC11567841
- DOI: 10.1002/alz.14219
REAL AD-Validation of a realistic screening approach for early Alzheimer's disease
Abstract
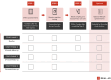
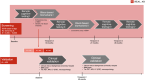
Early diagnosis is crucial to treatment success. This is especially relevant for Alzheimer's disease (AD), with its protracted preclinical phase. Most health care systems do not have the resources to conduct large-scale AD screenings in middle-aged individuals in need of novel AD treatment options and early, accurate diagnosis. Recent developments in blood-based biomarkers and remote cognitive testing offer novel, cost-effective, and scalable methods to detect cognitive and biomarker changes that may indicate early AD. In research cohorts, promising results have been reported, but these modalities have not been validated in population-based settings. The validation of a realistic screening approach for early Alzheimer's disease (REAL AD) study aims to validate the diagnostic and prognostic performance of the combined use of blood-based biomarkers and remote cognitive testing as a screening approach for early AD employing an existing health care infrastructure (the Swedish Västra Götaland Region Primary Healthcare). REAL AD aims to provide a concrete, individualized diagnostic framework, which could significantly improve AD prognosis. HIGHLIGHTS: In Sweden, most Alzheimer's disease (AD) diagnoses are made in primary care, where access to AD biomarkers is almost non-existent. Most health care systems have limited resources for the screening of middle-aged adults for early evidence of AD pathology. Blood-based biomarkers and remote cognitive testing offer novel, cost-effective, and scalable methods for detecting cognitive and biomarker changes that may indicate early AD. The REAL AD study aims to validate the diagnostic and prognostic performance of blood-based biomarkers and remote cognitive testing as a screening approach for early AD in an existing primary health care infrastructure in the Västra Götaland Region in Sweden. Studies such as REAL AD will play a vital role in helping to move the field toward concrete implementation of biomarkers in AD diagnostic workup at all care levels, eventually providing more comprehensive treatments options for the large and growing AD population, and for those at risk.
Keywords: Alzheimer's disease; blood‐based biomarkers; digital biomarker; primary care; screening.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
F.H., I.B., F.L., M.D., K.Q., and AM declare no conflicts of interest. A.L. serves as a consultant to Enigma Biomedical Group. S.K. has served on scientific advisory boards, as a speaker, and/or as consultant for Roche, Geras Solutions, Optoceutics, Biogen, and Bioarctic. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by Alzecure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and on advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers; has served on data‐monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), a part of the GU Ventures Incubator Program, outside the work presented in this article. M.S. has served on advisory boards for Roche and Novo Nordisk; received speaker honoraria from Bioarctic, Eisai, Genentech, Novo Nordisk, and Roche; and receives research support (to the institution) from Alzpath, Bioarctic, Novo Nordisk, and Roche. He is a co‐founder of Centile Bioscience and serves as Associate Editor with
Figures
References
-
- Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2023;19:658‐670. - PubMed
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS‐ADRDA Work Group under the auspices of department of health and human services task force on Alzheimer's Disease. Neurology. 1984;34:939‐944. - PubMed
-
- Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS‐ADRDA criteria. Lancet Neurol. 2007;6:734‐746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- #ADSF-21-831381-C/AD Strategic Fund and the Alzheimer's Association
- FO2024-0372/Swedish Brain Foundation
- AF-842471/the Alzheimerfonden
- AARF-22-972252/ALZ/Alzheimer's Association/United States
- Knut and Alice Wallenberg Foundation
- #AF-994551/Swedish Alzheimer Foundation
- Stiftelsen Hjalmar Svenssons Forskningsfond; and Stiftelsen Wilhelm och Martina Lundgrens vetenskapsfond
- Stiftelsen Demensfonden
- RS-2023-00263612/National Research Foundation of Korea
- La Fondation Recherche Alzheimer
- #AF-930351/Swedish Alzheimer Foundation
- R01 AG081394/AG/NIA NIH HHS/United States
- Familjen Rönströms Stiftelse
- Kirsten and Freddy Johansen Foundation
- #AF-939721/Swedish Alzheimer Foundation
- ALFGBG-81392/Swedish state
- 2019-02075/Swedish Research Council
- ZEN-21-848495/Alzheimer's Association 2021 Zenith
- #201809-2016862/Alzheimer's Drug Discovery Foundation
- Stiftelsen Psykiatriska Forskningsfonden
- Olav Thon Foundation
- AF-994900/Swedish Alzheimer Foundation
- Gun & Bertil Stohnes Stiftelse
- #ADSF-24-1284328-C/AD Strategic Fund and the Alzheimer's Association
- Erling-Persson Family Foundation
- JPND2019-466-236/European Union Joint Program for Neurodegenerative Disorders
- Sahlgrenska Academy at the University of Gothenburg
- UKDRI-1003/UK Dementia Research Institute at UCL
- Bluefield Project
- 860197/Marie Skłodowska-Curie
- #ADSF-21-831377-C/AD Strategic Fund and the Alzheimer's Association
- ALFGBG-965923/Swedish state
- AF-939825/the Alzheimerfonden
- VGFOUREG-995510/Västra Götaland Region R&D
- #ADSF-21-831376-C/AD Strategic Fund and the Alzheimer's Association
- #FO2022-0270/Hjärnfonden, Sweden
- #AF-968270/Swedish Alzheimer Foundation
- AF-737641/the Alzheimerfonden
- ALF GBG-771071/Swedish state
- #ALFGBG-71320/Swedish State Support for Clinical Research
- Stiftelsen för Gamla Tjänarinnor
- Cure Alzheimer's Fund
- JPND2021-00694/European Union Joint Programme - Neurodegenerative Disease Research
- R01 AG081394-01/NH/NIH HHS/United States
- 101053962/European Union's Horizon Europe research and innovation programme
- 2019-02075_15/Swedish Research Council
- AF-929959/the Alzheimerfonden
- FO2021-0311/Swedish Brain Foundation
- National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre
LinkOut - more resources
Full Text Sources
Medical

