Strategies for effective high pressure germination or inactivation of Bacillus spores involving nisin
- PMID: 39311577
- PMCID: PMC11505639
- DOI: 10.1128/aem.02299-23
Strategies for effective high pressure germination or inactivation of Bacillus spores involving nisin
Abstract
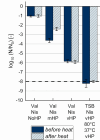
The major challenge in employing high pressure (HP) at moderate temperature for sterilization is the remarkable resistance of bacterial spores. High isostatic pressure can initiate spore germination, enabling subsequent inactivation under mild conditions. However, not all spores could be triggered to germinate under pressure at temperatures ≤80°C so far. In this study, germination treatment combinations were evaluated for Bacillus spores involving moderate HP (150 MPa, 37°C, 5 min), very HP (vHP, 550 MPa, 60°C, 2.5 or 9 min), simple and complex nutrient germinants [L-valine, L-alanine, and tryptic soy broth (TSB)], nisin, and incubation at atmospheric pressure (37°C). The most effective combinations for Bacillus subtilis resulted in a reduction of culturable dormant spores by 8 log10 units. The combinations involved nisin, a nutrient germinant (L-valine or TSB), a first vHP treatment (550 MPa, 60°C, 2.5 min), incubation at atmospheric pressure (37°C, 6 h), and a second vHP treatment (550 MPa, 60°C, 2.5 min). Such treatment combination with L-valine reduced Bacillus amyloliquefaciens spores by only 2 log10 units. B. amyloliquefaciens, thus, proved to be substantially more HP-resistant compared to B. subtilis, validating previous studies. Despite combining different germination mechanisms, complete germination could not be achieved for either species. The natural bacteriocin nisin did seemingly not promote HP germination initiation under chosen HP conditions, contrary to previous literature. Nevertheless, nisin might be beneficial to inhibit the growth of HP-germinated or remaining ungerminated spores. Future germination experiments might consider that nisin could not be completely removed from spores by washing, thereby affecting plate count enumeration.
Importance: Extremely resistant spore-forming bacteria are widely distributed in nature. They infiltrate the food chain and processing environments, posing risks of spoilage and food safety. Traditional heat-intensive inactivation methods often negatively affect the product quality. HP germination-inactivation offers a potential solution for better preserving sensitive ingredients while inactivating spores. However, the presence of ungerminated (superdormant) spores hampers the strategy's success and safety. Knowledge of strategies to overcome resistance to HP germination is vital to progress mild spore control technologies. Our study contributes to the evaluation and development of mild preservation processes by evaluating strategies to enhance the HP germination-inactivation efficacy. Mild preservation processes can fulfill the consumers' demand for safe and minimally processed food.
Keywords: Bacillus amyloliquefaciens; Bacillus subtilis; endospore; germination; high isostatic pressure; inactivation; nisin; superdormant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Characterization of high-pressure-treated Bacillus subtilis spore populations using flow cytometry - Shedding light on spore superdormancy at 550 MPa.Int J Food Microbiol. 2024 Sep 16;422:110812. doi: 10.1016/j.ijfoodmicro.2024.110812. Epub 2024 Jun 30. Int J Food Microbiol. 2024. PMID: 38970996
-
Isolation, stability, and characteristics of high-pressure superdormant Bacillus subtilis spores.Int J Food Microbiol. 2021 Apr 2;343:109088. doi: 10.1016/j.ijfoodmicro.2021.109088. Epub 2021 Feb 11. Int J Food Microbiol. 2021. PMID: 33621831
-
The combined effects of high pressure and nisin on germination and inactivation of Bacillus spores in milk.J Appl Microbiol. 2008 Jul;105(1):78-87. doi: 10.1111/j.1365-2672.2007.03722.x. Epub 2008 Jan 31. J Appl Microbiol. 2008. PMID: 18248377
-
Bacillus spore germination at moderate high pressure: A review on underlying mechanisms, influencing factors, and its comparison with nutrient germination.Compr Rev Food Sci Food Saf. 2021 Jul;20(4):4159-4181. doi: 10.1111/1541-4337.12789. Epub 2021 Jun 19. Compr Rev Food Sci Food Saf. 2021. PMID: 34147040 Review.
-
Mechanisms of endospore inactivation under high pressure.Trends Microbiol. 2013 Jun;21(6):296-304. doi: 10.1016/j.tim.2013.03.001. Epub 2013 Mar 26. Trends Microbiol. 2013. PMID: 23540831 Review.
Cited by
-
Revisiting bacterial spore germination in the presence of peptidoglycan fragments.J Bacteriol. 2025 Jul 24;207(7):e0014625. doi: 10.1128/jb.00146-25. Epub 2025 Jul 3. J Bacteriol. 2025. PMID: 40607799 Free PMC article.
References
-
- Setlow P, Johnson EA. 2019. Spores and their significance, p 23–63. In Food microbiology: fundamentals and frontiers. Wiley.
-
- Hu H, Balasubramaniam VM. 2024. High-pressure processing, p 531–551. In Encyclopedia of food safety, 2nd ed. Elsevier.
-
- Wells-Bennik MHJ, Eijlander RT, den Besten HMW, Berendsen EM, Warda AK, Krawczyk AO, Nierop Groot MN, Xiao Y, Zwietering MH, Kuipers OP, Abee T. 2016. Bacterial spores in food: survival, emergence, and outgrowth. Annu Rev Food Sci Technol 7:457–482. doi:10.1146/annurev-food-041715-033144 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

