Response to daratumumab-retreatment in patients with multiple myeloma
- PMID: 39311957
- PMCID: PMC11971056
- DOI: 10.1007/s00277-024-05991-7
Response to daratumumab-retreatment in patients with multiple myeloma
Abstract
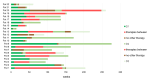
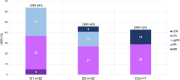
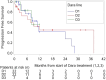
Daratumumab is an effective therapy in multiple myeloma (MM). We assessed whether daratumumab retreatment may re-induce significant responses and which patients do benefit the most. We hypothesized, that there is effective synergism between daratumumab and alternating antimyeloma drug combinations during retreatment and that retreatment is safe and effective. Here, we analyzed 293 consecutive MM patients receiving daratumumab at our institution from 2016 until 2023 retrospectively, and compared responses, side effects and survival of the first daratumumab treatment line and its retreatment. We identified 22/293 (8%) patients with daratumumab retreatment. These patients showed an advanced age and ISS/R-ISS stages, and ≥ 3 lines of prior antimyeloma therapy in 91%. Of note, the median durations of the first and subsequent daratumumab treatment were similarly long. We confirmed a therapy break between daratumumab lines as advantageous. Daratumumab retreatment was effective, with responses declining only gradually from its first use to subsequent first and second retreatment with 64%, 46% and 43%, respectively. Interestingly, comparable progression free survival rates were observed with 11.5, 12 months and not reached, respectively. Consistently, adverse events per daratumumab line did not increase. Our findings suggest that well-selected daratumumab-exposed MM patients may show rewarding responses to daratumumab retreatment, the more with alternating antimyeloma combinations, initial good response and CD38-antibody-treatment pauses, thereby proving CD38-antibody-retreatment as feasible, effective and non-toxic. Confirmatory studies are required to further validate our results.
Keywords: CD38-antibody; Multiple myeloma; Retreatment.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study is registered at Freiburg Register of Clinical Trials under protocol FRKS004511 and was approved by the Ethics Committee of the University of Freiburg (EV 23-1110_1-S1). It was performed according to the guidelines of the Declaration of Helsinki and Good Clinical Practice. Informed consent: All patients gave their written informed consent for institutionally initiated research studies in accordance with the institutional review board guidelines. Competing interests: RW consulted for Amgen, BMS/Celgene, Janssen, Novartis, Kite/Gilead, Pfizer, Sanofi and Takeda, he received research funding from Janssen and Sanofi, and travel support from Janssen, Kite/Gilead, Pfizer and BMS. The other authors declare no competing financial interest related to this study.
Figures

References
-
- Global Cancer Observatory Cancer Today. International Agency for Research on Cancer. https://gco.iarc.fr/today
-
- Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, Lust J, McCurdy A, Russell SJ, Zeldenrust SR, Kyle RA, Rajkumar SV (2014) Continued improvement in Survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 28(5):1122–1128. 10.1038/leu.2013.313 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

