Single-Cell Transcriptomic Analysis Identifies Senescent Osteocytes That Trigger Bone Destruction in Breast Cancer Metastasis
- PMID: 39312185
- PMCID: PMC11611663
- DOI: 10.1158/0008-5472.CAN-24-0857
Single-Cell Transcriptomic Analysis Identifies Senescent Osteocytes That Trigger Bone Destruction in Breast Cancer Metastasis
Abstract
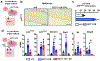
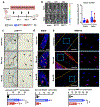
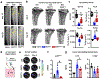
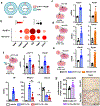
Breast cancer bone metastases increase fracture risk and are a major cause of morbidity and mortality among women. Upon colonization by tumor cells, the bone microenvironment undergoes profound reprogramming to support cancer progression, which disrupts the balance between osteoclasts and osteoblasts and leads to bone lesions. A deeper understanding of the processes mediating this reprogramming could help develop interventions for treating patients with bone metastases. Here, we demonstrated that osteocytes (Ot) in established breast cancer bone metastasis develop premature senescence and a distinctive senescence-associated secretory phenotype (SASP) that favors bone destruction. Single-cell RNA sequencing identified Ots from mice with breast cancer bone metastasis enriched in senescence, SASP markers, and pro-osteoclastogenic genes. Multiplex in situ hybridization and artificial intelligence-assisted analysis depicted Ots with senescence-associated satellite distension, telomere dysfunction, and p16Ink4a expression in mice and patients with breast cancer bone metastasis. Breast cancer cells promoted Ot senescence and enhanced their osteoclastogenic potential in in vitro and ex vivo organ cultures. Clearance of senescent cells with senolytics suppressed bone resorption and preserved bone mass in mice with breast cancer bone metastasis. These results demonstrate that Ots undergo pathological reprogramming by breast cancer cells and identify Ot senescence as an initiating event triggering lytic bone disease in breast cancer metastases. Significance: Breast cancer cells remodel the bone microenvironment by promoting premature cellular senescence and SASP in osteocytes, which can be targeted with senolytics to alleviate bone loss induced by metastatic breast cancer. See related commentary by Frieling and Lynch, p. 3917.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures




Update of
-
Single-cell Transcriptome Analysis Identifies Senescent Osteocytes as Contributors to Bone Destruction in Breast Cancer Metastasis.Res Sq [Preprint]. 2024 Mar 14:rs.3.rs-4047486. doi: 10.21203/rs.3.rs-4047486/v1. Res Sq. 2024. Update in: Cancer Res. 2024 Dec 2;84(23):3936-3952. doi: 10.1158/0008-5472.CAN-24-0857. PMID: 38558984 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
- Seeds of Science/Winthrop P. Rockefeller Cancer Institute, University of Arkansas for Medical Sciences (Winthrop P. Rockefeller Cancer Institute, UAMS)
- P20 GM125503/GM/NIGMS NIH HHS/United States
- U01 AG075227/AG/NIA NIH HHS/United States
- R01 CA241677/CA/NCI NIH HHS/United States
- R01 CA209882/CA/NCI NIH HHS/United States
- P20GM125503/National Institute of General Medical Sciences (NIGMS)
- F31 CA284655/CA/NCI NIH HHS/United States
- F31CA284655/National Cancer Institute (NCI)
- R37CA251763/National Cancer Institute (NCI)
- P20 GM103625/GM/NIGMS NIH HHS/United States
- TA1154/1-2 and TA1154/2-2/German Research Foundation
- R37 CA251763/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

