CDS2 expression regulates de novo phosphatidic acid synthesis
- PMID: 39312194
- PMCID: PMC11555650
- DOI: 10.1042/BCJ20240456
CDS2 expression regulates de novo phosphatidic acid synthesis
Abstract
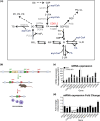
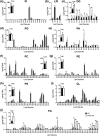
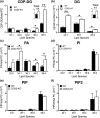
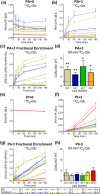
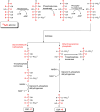
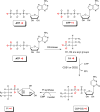
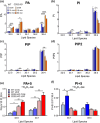
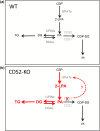
CDS enzymes (CDS1 and 2 in mammals) convert phosphatidic acid (PA) to CDP-DG, an essential intermediate in the de novo synthesis of PI. Genetic deletion of CDS2 in primary mouse macrophages resulted in only modest changes in the steady-state levels of major phospholipid species, including PI, but substantial increases in several species of PA, CDP-DG, DG and TG. Stable isotope labelling experiments employing both 13C6- and 13C6D7-glucose revealed loss of CDS2 resulted in a minimal reduction in the rate of de novo PI synthesis but a substantial increase in the rate of de novo PA synthesis from G3P, derived from DHAP via glycolysis. This increased synthesis of PA provides a potential explanation for normal basal PI synthesis in the face of reduced CDS capacity (via increased provision of substrate to CDS1) and increased synthesis of DG and TG (via increased provision of substrate to LIPINs). However, under conditions of sustained GPCR-stimulation of PLC, CDS2-deficient macrophages were unable to maintain enhanced rates of PI synthesis via the 'PI cycle', leading to a substantial loss of PI. CDS2-deficient macrophages also exhibited significant defects in calcium homeostasis which were unrelated to the activation of PLC and thus probably an indirect effect of increased basal PA. These experiments reveal that an important homeostatic response in mammalian cells to a reduction in CDS capacity is increased de novo synthesis of PA, likely related to maintaining normal levels of PI, and provides a new interpretation of previous work describing pleiotropic effects of CDS2 deletion on lipid metabolism/signalling.
Keywords: CDP-DG; CDS2; lipidomics; metabolism; phosphatidic acids; phosphatidylinositol.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures



References
-
- Blunsom, N.J., Gomez-Espinosa, E., Ashlin, T.G. and Cockcroft, S. (2017) Mitochondrial CDP-diacylglycerol synthase activity is due to the peripheral protein, TAMM41 and not due to the integral membrane protein, CDP-diacylglycerol synthase 1. Biochim. Biophys. Acta 1863, 284–298 10.1016/j.bbalip.2017.12.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

