Early Addition of Selexipag to Double Therapy for Pulmonary Arterial Hypertension
- PMID: 39312239
- PMCID: PMC11420696
- DOI: 10.1001/jamanetworkopen.2024.34691
Early Addition of Selexipag to Double Therapy for Pulmonary Arterial Hypertension
Abstract
Importance: A subgroup analysis of a randomized clinical trial established the efficacy of selexipag plus background therapy (monotherapy or double oral therapy [DOT]) vs placebo plus background therapy and found that the addition of selexipag within 6 months had an added benefit. However, the timing of selexipag addition to DOT and the incremental benefit in clinical practice is not well studied.
Objective: To compare triple oral therapy (TOT) consisting of selexipag, endothelin receptor antagonist (ERA), and phosphodiesterase type 5 inhibitor (PDE5i) vs DOT consisting of ERA and PDE5i.
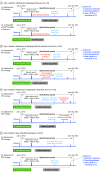
Design, setting, and participants: This comparative effectiveness study was conducted using data from the US Komodo claims database to emulate a randomized trial. Patients aged 18 years or older with pulmonary arterial hypertension (PAH) treated with ERA plus PDE5i with records from July 2015 through June 2022 were duplicated to TOT and DOT and artificially censored when observed treatment deviated from assigned treatment. Hypothetical randomization was emulated using inverse probability of treatment weighting, and the study accounted for censoring-induced selection bias using inverse probability of censoring weighting. A pooled logistic model estimated the per-protocol difference between treatment groups. Data were analyzed from November 2022 through July 2023.
Interventions: TOT (addition of selexipag within 3, 6, and 12 months of initiating DOT) vs DOT.
Main outcomes and measures: Adjusted risk of all-cause hospitalization, PAH-related hospitalization, and PAH-related disease progression over a 2-year follow-up.
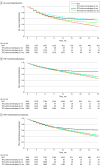
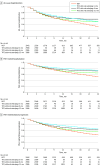
Results: A total of 2966 patients with PAH (mean [SD] age, 54.3 [14.0] years; 2125 female [71.6%]) met eligibility criteria. Adding selexipag within 6 months of ongoing DOT was associated with a reduction in risk for all-cause hospitalization (adjusted hazard ratio [aHR], 0.82; 95% CI, 0.72-0.94), PAH-related hospitalization (aHR, 0.81; 95% CI, 0.70-0.95), and PAH-related progression (aHR, 0.82; 95% CI, 0.70-0.95) vs DOT alone. There were no associations if selexipag was initiated within 12 months for all-cause hospitalization, PAH-related hospitalization, or PAH-related disease progression. The association remained with a greater decrease in risk for disease progression vs DOT for selexipag initiation within 3 months (aHR, 0.74; 95% CI, 0.61-0.90).
Conclusions and relevance: This study found that early selexipag addition to ERA plus PDE5i was associated with a reduction in risk of hospitalization and disease progression. These findings suggest that delays in selexipag initiation likely contribute to suboptimal patient and health system outcomes.
Conflict of interest statement
Figures
Comment in
-
Novel Approach to Evaluate the Role of Selexipag in Pulmonary Arterial Hypertension.JAMA Netw Open. 2024 Sep 3;7(9):e2434644. doi: 10.1001/jamanetworkopen.2024.34644. JAMA Netw Open. 2024. PMID: 39312243 No abstract available.
Similar articles
-
Real-Life Experience with Selexipag as an Add-On Therapy to Oral Combination Therapy in Patients with Pulmonary Arterial or Distal Chronic Thromboembolic Pulmonary Hypertension: A Retrospective Analysis.Lung. 2019 Jun;197(3):353-360. doi: 10.1007/s00408-019-00222-7. Epub 2019 Apr 8. Lung. 2019. PMID: 30963265
-
Patient and disease characteristics of the first 500 patients with pulmonary arterial hypertension treated with selexipag in real-world settings from SPHERE.J Heart Lung Transplant. 2021 Apr;40(4):279-288. doi: 10.1016/j.healun.2021.01.006. Epub 2021 Jan 15. J Heart Lung Transplant. 2021. PMID: 33526303
-
Three- Versus Two-Drug Therapy for Patients With Newly Diagnosed Pulmonary Arterial Hypertension.J Am Coll Cardiol. 2021 Oct 5;78(14):1393-1403. doi: 10.1016/j.jacc.2021.07.057. J Am Coll Cardiol. 2021. PMID: 34593120 Clinical Trial.
-
Effectiveness and safety of endothelin receptor antagonists, alone and in combination therapy, in the pulmonary arterial hypertension-connective tissue disease subtype: A systematic review and meta-analysis.Int J Rheum Dis. 2020 Oct;23(10):1276-1287. doi: 10.1111/1756-185X.13916. Epub 2020 Jul 21. Int J Rheum Dis. 2020. PMID: 32691518
-
Selexipag for the treatment of pulmonary arterial hypertension.Expert Rev Respir Med. 2021 May;15(5):583-595. doi: 10.1080/17476348.2021.1866990. Epub 2020 Dec 31. Expert Rev Respir Med. 2021. PMID: 33382345 Review.
References
-
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. Published online September 1, 2020. doi:10.1177/2045894020950186 - DOI - PMC - PubMed
-
- Coghlan JG, Channick R, Chin K, et al. . Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs. 2018;18(1):37-47. doi:10.1007/s40256-017-0262-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

